Figures & data
Figure 1. LAT associates with the active form of Lck. (A) Jurkat T cells (107) were stimulated with 5 μg/ml PHA for the times indicated and Lck was immunoprecipitated. Immune complexes were analyzed with anti-LAT (upper panel) and anti-Lck (lower panel) antibodies. (B) Lck immunoprecipitations from Jurkat T cells stimulated with beads coated with anti-CD3/CD28 antibodies for the indicated times. (C) 5 × 106 purified T cells from a healthy volunteer and a patient with active SLE were left unstimulated or stimulated with anti-CD3/CD28. Immunoprecipitated Lck and associated LAT were detected with specific antibodies. The experiment shown is one out of three performed with T cells from different donors with similar result.
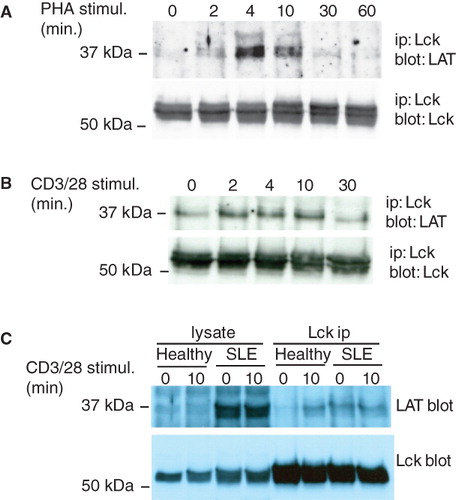
Figure 2. In vitro produced LAT and Y505F-Lck do not interact. LAT and Y505F-Lck proteins were produced in an in vitro transcription/translation system and successful production was established by Western blotting (lysate). Aliquots of the mix were used for Lck and LAT immonoprecipitations and immune complexes were analysed for the presence of associated proteins (ips). The star in the immunoprecipitations panel indicates the migration distance of the heavy chain of the immunoprecipitating antibody.
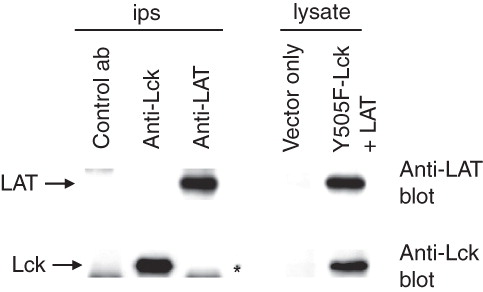
Figure 3. Generation of LAT mutants. (A) Schematic representation of the LAT mutants generated for this study. (B) COS cells were transfected with the indicated plasmids and potential association of expressed proteins was assessed in co-immunoprecipitation experiments. Equivalent expression of proteins was determined by Western blotting of cell lysate aliquots.
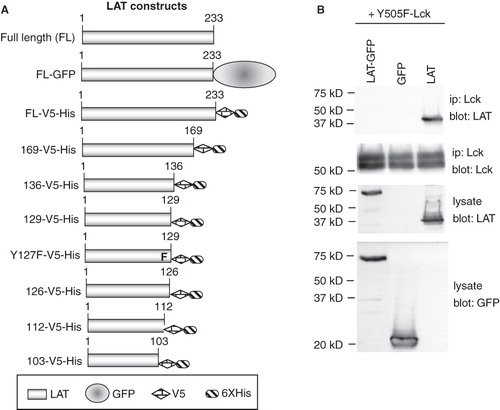
Figure 4. A string of acidic residues mediate the interaction of LAT with active Lck. (A), (B), and (C) COS cells were singly transfected or co-transfected with the indicated LAT mutants and Y505F-Lck. Comparable expression of proteins was assessed by Western blotting equivalent aliquots of cell lysates with anti-Lck and anti-LAT antibodies (lysate). Association of the various LAT mutants with Y505F-Lck was determined by Western blotting Lck immunoprecipitations with anti-LAT antibodies (Lck ip). The levels of immunoprecipitated Lck from each sample were determined by immunoblotting the same membrane with anti-Lck antibodies.
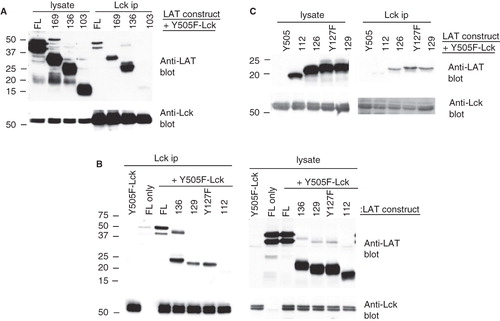
Table I. Summary of LAT mutant interaction with Y505F Lck.
Table II. Amino acid sequence of the corresponding LAT domains from various species deposited in the NCBI databank. Negatively charged amino acids are in bold.