Figures & data
Figure 1. DHHC2 and DHHC4 siRNA treatment causes Lck membrane dislocation. Where indicated, ‘pool of four’ siRNA oligos directed towards DHHC2, 4, 15 and 16 were introduced by Amaxa nucleofection into Jurkat T cells at the concentrations indicated. 72 h later, cells were fractionated into a P100 particulate membrane fraction and an S100 soluble cytosolic fraction, the proteins separated by SDS-PAGE and endogenous Lck, actin and calnexin in the fractions were detected by Western blotting. Actin was used as a loading control, and absence of calnexin in the S100 fraction serves as a control for the fractionation.
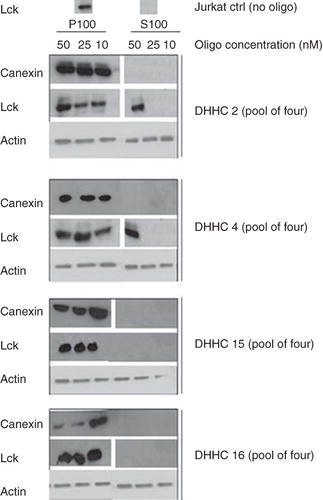
Figure 2. Tools for DHHC protein analysis and membrane association of DHHC proteins. (A) Expression vectors encoding full length DHHC2, 4 and 16 fused to CFP at their C-terminus and CFP alone, were transiently transfected into HEK293A cells. 24 h post transfection, the expression of the DHHC-CFP proteins and CFP were analyzed in total cell lysates by Western blotting using anti-GFP antibody. (B) Specificity of the anti-DHHC2 antibody. DHHC2 (both endogenous and exogenous CFP-fused) was analyzed by Western blotting in cell lysates from HEK293A cells transiently transfected for 24 h with DHHC2-CFP, DHHC4-CFP and CFP. The anti-DHHC2 antibody recognizes endogenous DHHC2 in all lanes and DHHC2-CFP without cross-reactivity to DHHC4. (C) Distribution of DHHC-CFPs and CFP alone between the P100 particulate membrane fraction and the S100 soluble cytosolic fraction of HEK293A cells transiently transfected for 24 h as detected by Western blotting using an anti-GFP antibody.
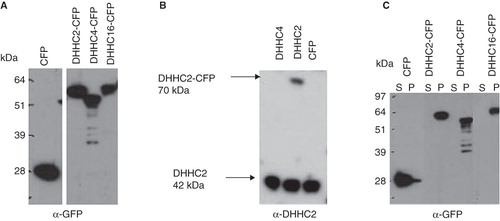
Figure 3. Enhanced S-acylation of LckN10-GFP by cotransfected DHHC2-CFP. HEK293A cells were co-transfected with LckN10-GFP and DHHC2-CFP or DHHC4-CFP as indicated. (A) Autoradiography of total cell lysates separated on NuPage gels following metabolic labelling with 3H-palmitate for 2 h. Upper panel – long exposure (21 days) showing 3H-palmitate labelling of transfected DHHC2- and DHHC4-CFP. Lower panel – 2 day exposure showing 3H-palmitate labelling of transfected LckN10-GFP. Numbers represent relative acylation levels normalized to control. (B) Western blotting using anti-GFP antibody detecting LckN10-GFP and DHHC2- and DHHC4-CFP levels in total lysates. The positions of bands corresponding to DHHC2-CFP, DHHC4-CFP and LckN10-GFP are indicated.
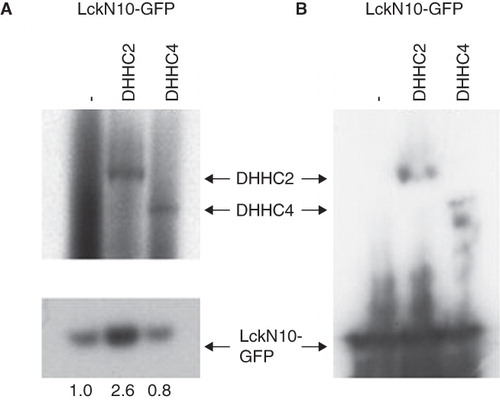
Figure 4. Decreased Lck S-acylation after DHHC2 siRNA treatment. (A) Quantitative real-time PCR was used to assess DHHC2 and DHHC4 mRNA expression in Jurkat cells transfected with Accell siRNA directed against either DHHC2 (siDHHC2) or DHHC4 (siDHHC4). Relative expression compared to Jurkat T cells transfected with a control siRNA (non-targeting) is shown. Error bars are SEM. Results are representative of two independent experiments. (B) Levels of DHHC2 in total protein lysates from Jurkat T cells treated with Accell siRNA against DHHC2 or DHHC4 or without siRNA oligos. DHHC2 was detected by Western blotting using the anti-DHHC2 antibody and detection of tubulin with anti-tubulin antibody was used as a loading control. (C) Total protein lysates from Jurkat T cells Ju or J.Cam1.6 cells treated for 72 h with siRNA oligos directed towards DHHC2 or control siRNA. The cells were labelled with 3H-palmitic acid to show S-acylation. Lck is one of the most abundant S-acylated proteins in Jurkat T cells and its position on the gel is indicated; absence of a corresponding band in Lck-negative J.Cam1.6 cells validates the assignment of the Lck band.
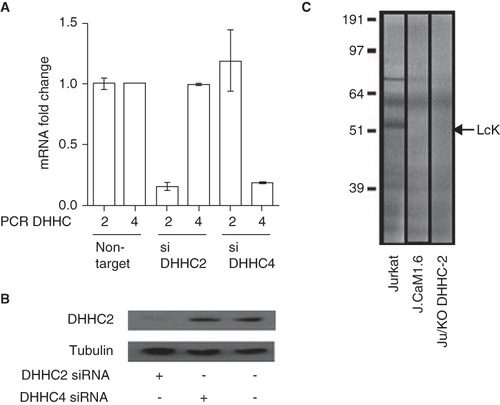
Figure 5. Subcellular localization of DHHC-CFPs and endogenous DHHC2. (A) Confocal microscopy images of HeLa cells transiently expressing the indicated DHHC-CFPs or CFP alone. Scale bars = 10 μm. (B) Confocal microscopy images of HeLa cells transiently expressing DHHC2-CFP with Golgi apparatus indicated by immunofluorescence using anti-Golgin 97 and ER using anti-calnexin antibodies. Scale bars = 10 μm. (C) Confocal microscopy images of HEK293A cells immunostained for DHHC2, Rab5 as a marker of early endosomes, Golgin 97 as a marker for the Golgi apparatus and calreticulin as a marker for the ER.
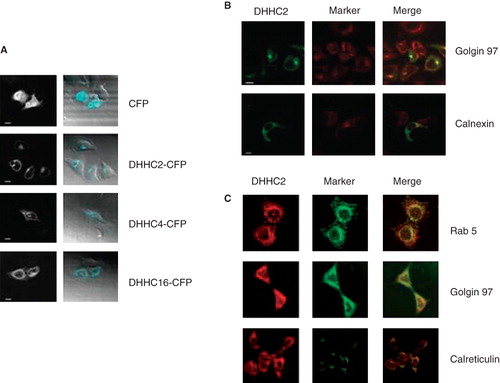
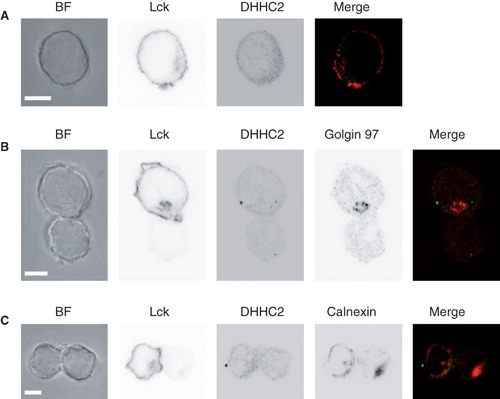
Figure 6. DHHC2 localization in resting and activated Jurkat T cells. (A) Brightfield (BF) and confocal microscopy images of DHHC2 and Lck detected by immunostaining in Jurkat T cells. Scale bars = 5 μm. (B) and (C) BF and confocal immunofluorescence images of Jurkat T cells (top in B, left in C; marked with anti-Lck) conjugated with SEE-loaded Raji B cells. Greyscale images are shown for clarity. In (B) the Golgi apparatus is visualized by staining with anti-Golgin 97 and in (C) the ER is visualized by staining with anti-calnexin. Merged images are also shown; green – DHHC2; red – Golgin 97 (B) or calnexin (C). Accumulation of Lck at the T-B cell interface confirms productive formation of the immunological synapse. Images are representative of n = 23 (B) and n = 24 (C) cell pairs. Scale bars = 5 μm.