Figures & data
Figure 1. Detection of non-bilayer phospholipid arrangements on liposomes by freeze-fracture electron microscopy and 31P NMR spectroscopy. Liposomes made of phosphatidylcholine/cardiolipin (2:1 molar ratio) were incubated at 37°C for 30 min with: TS buffer (top panels) or the non-bilayer phospholipid arrangements inducers: 5 mM MnCl2 (middle panels), or 3 mM chlorpromazine (bottom panels). (A) Freeze-fracture electron microscopy. The black arrow in the top panel indicates the shadow direction. Small white arrows show non-bilayer phospholipid arrangements. The solid line represents 100 nm in all micrographs. (B) 31P NMR spectra were attained at 37°C using 70 μmol of liposomes incubated with TS buffer or the same non-bilayer phospholipid arrangements inducers as above. The graph number 1 in the middle panel corresponds to phosphatidylcholine/cardiolipin (2:1) liposomes and the graph number 2 corresponds to phosphatidylcholine liposomes as a control. The broken line indicates the reference values for changes in ppm of NMR. The same liposome preparations were used for these determinations and for the immunological assays.
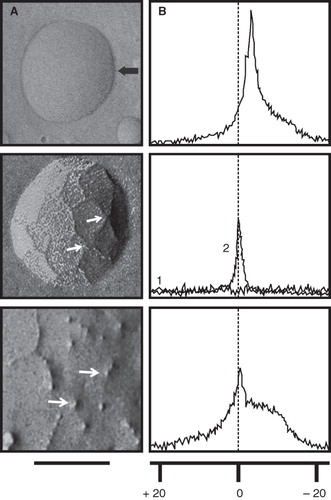
Figure 2. Detection of non-bilayer phospholipid arrangements on liposomes by flow cytometry. Liposomes made of phosphatidylcholine (PC)/phosphatidic acid (PA) (2:1 molar ratio) or phosphatidylcholine/phosphatidylserine (PS) (4:1) were incubated at 37°C for 30 min with the indicated concentrations of Mn2+ or chlorpromazine. Changes in bilayer complexity (SSC) and liposomal aggregation (FSC) were evaluated. Bilayer complexity (SSC) is represented in histograms (red lines represent liposomes alone; blue lines represent liposomes with Mn2+ or with chlorpromazine; the D value of the Kolmogorov-Smirnov test is indicated); bilayer complexity and liposomal aggregation are represented in density plots.
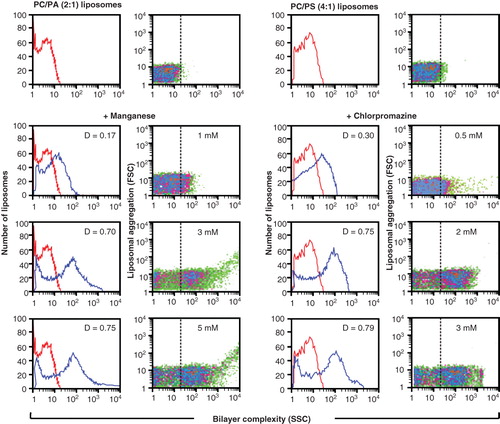
Figure 3. H308-AP monoclonal antibody binds non-bilayer phospholipid arrangements induced by Mn2+, chlorpromazine and procainamide. Liposomes made of phosphatidylcholine (PC)/phosphatidic acid (PA) (2:1 molar ratio), phosphatidylcholine/cardiolipin (CL) (2:1) or dipalmitoylphosphatidylcholine (DPPC)/phosphatidylcholine/dipalmitoylphosphatidic acid (DPPA) (1.2:0.8:1) were used alone or in the presence of 5 mM MnCl2, 3 mM chlorpromazine (CPZ), 8 mM procainamide (PCM), 5 mM MgCl2, 5 mM BaCl2, 1.5 mM NaCl2 or 5 mM chloroquine (CQ). Liposomes were stained with FITC-labelled H308-AP monoclonal antibody or with an isotype control. Changes in liposomal fluorescence, bilayer complexity (SSC), and liposomal aggregation (FSC) were evaluated. One experiment representative of three is shown.
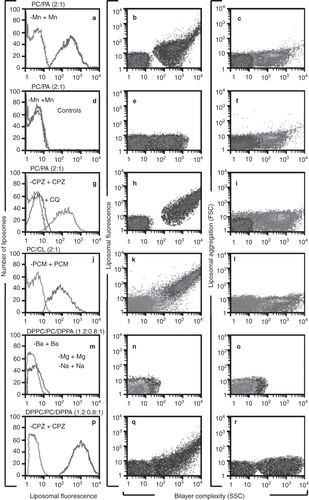
Figure 4. Phosphorylcholine and glycerolphosphorylcholine prevent the binding of H308-AP to non-bilayer phospholipid arrangements. H308-APmAb was incubated with phosphate, phosphorylcholine, glycerolphosphorylcholine, phosphorylserine, glycerolphosphorylserine, glycerolphosphate or glycerolphosphorylglycerol (the chemical structure of some of these haptens is shown). The ability of the H308-AP monoclonal antibody to bind phosphatidylcholine/cardiolipin liposomes with chlorpromazine-induced non-bilayer phospholipid arrangements was assessed by liposomal ELISA. As a negative control, we used liposomes without non-bilayer phospholipid arrangements.
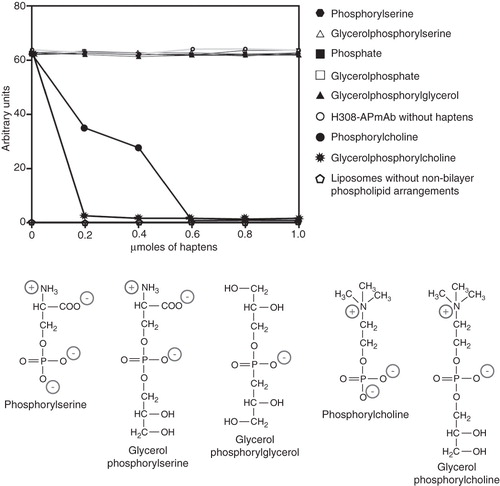
Figure 5. Liposomes with non-bilayer phospholipid arrangements induced by Mn2+ cause an autoimmune disease resembling human lupus in mice. (A) and (B) Representative photographs of 6-month-old female BALB/c mice treated with phosphatidylcholine/phosphatidic acid liposomes with non-bilayer phospholipid arrangements induced by Mn2+, showing facial lesions (mice are identified by marks made with a picric acid solution, which show as yellow colouring). (C) Glomeruli with mesangial hypercellularity (asterisks) and thickening of capillary walls (arrows). (D) Skin from alopecic areas showing epidermal atrophy (arrows), and widening of hair bulb fibrous sheath (asterisks) with total disorganization of matrical cells. In contrast, there are not histological abnormalities in kidney (E) or skin (F) from control mice treated with phosphatidylcholine/phosphatidic acid liposomes (H/E, 40× in all panels).
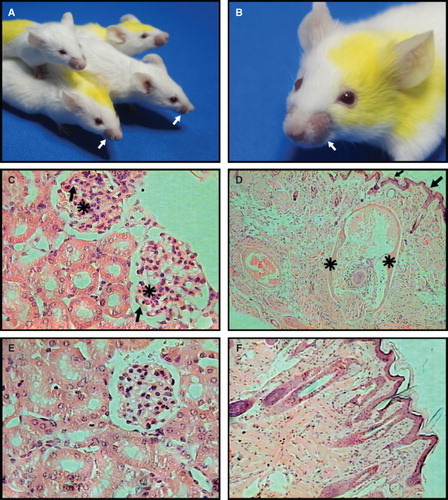
Figure 6. Sera of mice with the autoimmune disease bind specifically to non-bilayer phospholipid arrangements. The serum of a mouse with the autoimmune disease caused by Mn2+-treated phosphatidylcholine/phosphatidic acid liposomes was assayed by flow cytometry, using Mn2+-treated phosphatidylcholine/phosphatidic acid liposomes (A) or chlorpromazine-treated phosphatidylcholine/phosphatidic acid liposomes (B). The serum of a mouse with the autoimmune disease caused by chlorpromazine-treated phosphatidylcholine/phosphatidic acid liposomes was assayed by flow cytometry, using chlorpromazine-treated phosphatidylcholine/phosphatidic acid liposomes (C), and Mn2+-treated phosphatidylcholine/phosphatidic acid liposomes (D). Changes in liposomal fluorescence were evaluated. Filled histograms represent the serum of a mouse with the disease; empty histograms represent pre-immune serum.
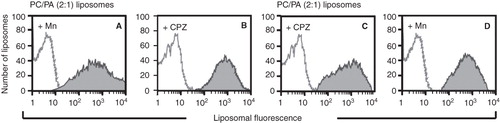
Figure 7. Sera of mice with the autoimmune disease have antibodies that bind non-bilayer phospholipid arrangements, as well as anti-cardiolipin, anti-histone and lupus anticoagulant antibodies. Mice (10 per group) received phosphatidylcholine/phosphatidic acid liposomes alone (controls) or treated with Mn2+, chlorpromazine or procainamide. The titers of antibodies that bind non-bilayer phospholipid arrangements (A), anti-cardiolipin antibodies (B), anti-histone antibodies (C) and lupus anticoagulant antibodies (D) were determined before the administration of liposomes, and 1, 2 and 3 months after liposome administration. Data were analysed by two-way ANOVA with Bonferroni post-tests.
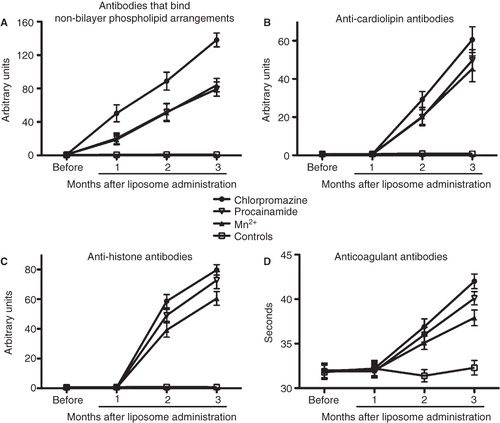
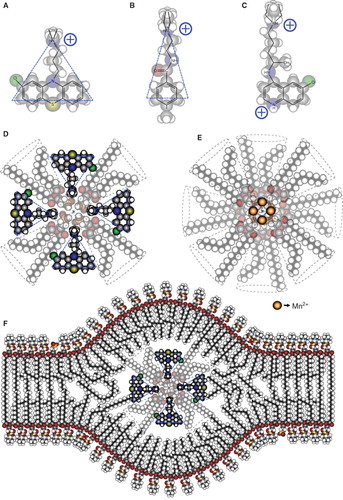
Figure 8. Molecular organization of the non-bilayer phospholipid arrangements that induce an autoimmune disease resembling human lupus in mice. Chemical structure and molecular shape of chlorpromazine (A), procainamide (B) and chloroquine (C). (D) Inverted micelle with chlorpromazine. (E) Inverted micelle with Mn2+. (F) Inverted micelle with chlorpromazine inserted in the bilayer arrangement of the liposome, forming the non-bilayer phospholipid arrangement. The bilayer is mainly formed by phosphatidylcholine, whose polar regions are exposed on the zones of the lipid bilayer where the inverted micelle is inserted. The increased exposure facilitates the production of antibodies against these regions.