Figures & data
Table I. Tissue distribution and functional roles of the 13 human AQPs. The Table summarizes the distribution and function of AQPs in specific cells and tissues of the human body. Immunohistochemistry has revealed the diversity of AQP distribution within cells and tissues. For example, AQPs 1, 2, 3, 4, 6, 7, 8, 9, 10 and 11 have all been shown to be expressed in tissues of the kidney whereas AQP0 is almost exclusively expressed in lens epithelial cells. AQP11 expression in leucocytes may explain the immunohistochemical identification of AQP11 in other tissues.
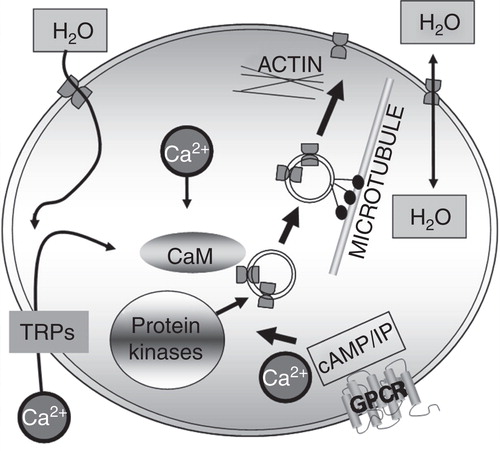
Table II. Triggers of aquaporin translocation to the plasma membrane (PM). The Table summarizes known stimuli of the dynamic translocation of AQPs, where possible avoiding observations solely linked to water permeability (WP) and/or transcription/translation. The experimental system used to determine the AQP translocation is included for each trigger.
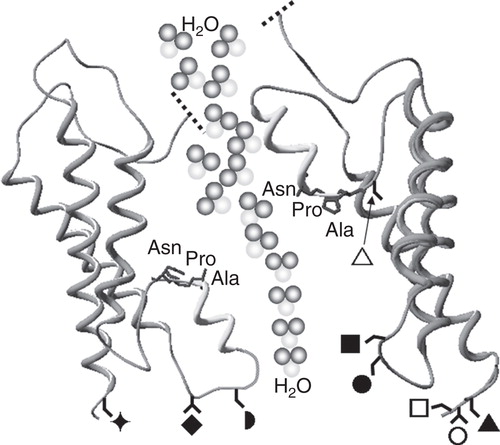
Table III. Intracellular components involved in the translocation of specific aquaporins to the plasma membrane (PM). The Table summarizes the components of dynamic translocation of AQPs avoiding, where possible, observations solely linked to water permeability (WP) and/or transcription/translation. Arrows indicate the direction of the effect. The experimental system used to determine the AQP translocation is included for each trigger and the residue that the component acts upon is also included where known. †Residues in PKC. *AQP2 is not added in more detail than PKA-linked as this has been very well reviewed elsewhere (Valenti et al. Citation2005).
= Ser111 and
= Ser180 in AQP4;
= Ser152 in AQP5;
= Ser11 and ▵ = Ser222 in AQP9.