Figures & data
Figure 1. A schematic model of the hypothalamic–pituitary–adrenal (HPA) axis. CRF plays a central role in controlling stress responses. CRF, produced in the hypothalamic PVN, stimulates ACTH production from the corticotrophs of the anterior pituitary (AP). ACTH then stimulates glucocorticoid release from the adrenal glands. Glucocorticoids, in turn, inhibits both CRF production in hypothalamus and the ACTH production in the AP.
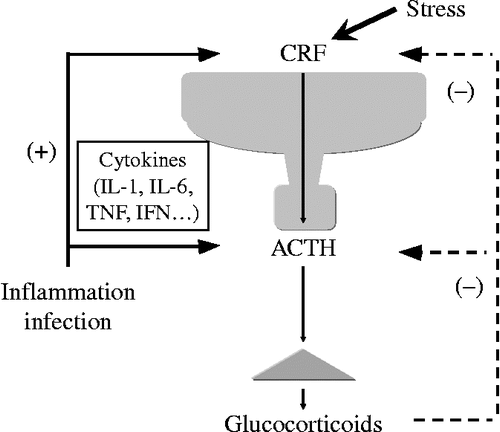
Figure 2. Plasma ACTH concentrations of WT and CRF KO. The basal ACTH concentrations were determined under pentobarbital anesthesia (n = 6–7). ACTH concentrations under pain stress were determined after tail-pinched stress for 15 s (n = 7–8); NS, not significant; *P < 0.05 and **P < 0.01 (compared between the two values indicated). [Reproduced with permission from Fukuda et al. (Citation2004). Copyright © Elsevier.]
![Figure 2. Plasma ACTH concentrations of WT and CRF KO. The basal ACTH concentrations were determined under pentobarbital anesthesia (n = 6–7). ACTH concentrations under pain stress were determined after tail-pinched stress for 15 s (n = 7–8); NS, not significant; *P < 0.05 and **P < 0.01 (compared between the two values indicated). [Reproduced with permission from Fukuda et al. (Citation2004). Copyright © Elsevier.]](/cms/asset/f19ac492-eae1-43d4-9b2c-334875525781/ists_a_536279_f0002_b.gif)
Figure 4. Effects of PACAP on cAMP production in hypothalamic 4B cells. Cells were pre-incubated for 20 min with the medium containing 0.1 mM 3-isobutyl-1-methylxanthine, followed by the addition of forskolin (Fsk) or PACAP. The level of intracellular cAMP was measured by cAMP EIA; *P < 0.05 and **P < 0.005 (compared with control (C)). [Reproduced with permission from Kageyama et al. (Citation2007). Copyright © Society for Endocrinology.]
![Figure 4. Effects of PACAP on cAMP production in hypothalamic 4B cells. Cells were pre-incubated for 20 min with the medium containing 0.1 mM 3-isobutyl-1-methylxanthine, followed by the addition of forskolin (Fsk) or PACAP. The level of intracellular cAMP was measured by cAMP EIA; *P < 0.05 and **P < 0.005 (compared with control (C)). [Reproduced with permission from Kageyama et al. (Citation2007). Copyright © Society for Endocrinology.]](/cms/asset/aab83192-5800-47c9-bb39-8e45de327412/ists_a_536279_f0004_b.gif)
Figure 5. Effects of CRE deletion on forskolin-induced CRF 5′-promoter activity in 4B cells. (A) Schematic representation of CRE in the CRF promoter. (B) Cells were transfected with full-length (CRF-907luc), deleted (CRF-220luc or CRF-233luc), or mutant (CRF-233Mtluc) promoter constructs then incubated for 2 h with 10 μM forskolin alone (Fsk) or vehicle (C); *P < 0.05 (compared with forskolin alone [Fsk] in CRF-907luc transfected cells) and +P < 0.05 (compared with each control). [Reproduced with permission from Kageyama et al. (Citation2008). Copyright © Editrice Kurtis srl.]
![Figure 5. Effects of CRE deletion on forskolin-induced CRF 5′-promoter activity in 4B cells. (A) Schematic representation of CRE in the CRF promoter. (B) Cells were transfected with full-length (CRF-907luc), deleted (CRF-220luc or CRF-233luc), or mutant (CRF-233Mtluc) promoter constructs then incubated for 2 h with 10 μM forskolin alone (Fsk) or vehicle (C); *P < 0.05 (compared with forskolin alone [Fsk] in CRF-907luc transfected cells) and +P < 0.05 (compared with each control). [Reproduced with permission from Kageyama et al. (Citation2008). Copyright © Editrice Kurtis srl.]](/cms/asset/1a08b529-388a-4a0f-8f43-a8c476043a76/ists_a_536279_f0005_b.gif)
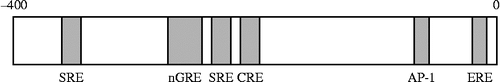
Figure 6. Effects of nGRE or SRE deletion on the dexamethasone suppression of CRF 5′-promoter activity in 4B cells. (A) Schematic representation of nGRE or SRE in the CRF promoter. (B) Cells transfected with a full-length (CRF-907luc), deleted (CRF-233luc, CRF-248luc, or CRF-278luc), or mutant (CRF-278Mtluc or CRF-248Mtluc) promoter construct, were pre-incubated for 30 min with the medium containing dexamethasone (Dex, 100 nM) or vehicle (C), then incubated for 2 h with the medium containing 10 μM forskolin. Data are presented as relative activity; luciferase activity in response to forskolin alone (C) was set at 100% in all transfected cells; *P < 0.05 (compared with forskolin alone [C] in all transfected cells), +P < 0.05 (compared with Dex in CRF-907luc), ++P < 0.05 (compared with Dex in CRF-907luc and CRF-278luc), and +++P < 0.05 (compared with Dex in CRF-907luc, CRF-278luc, and CRF-248luc). [Reproduced with permission from Kageyama et al. (Citation2008). Copyright © Editrice Kurtis srl.]
![Figure 6. Effects of nGRE or SRE deletion on the dexamethasone suppression of CRF 5′-promoter activity in 4B cells. (A) Schematic representation of nGRE or SRE in the CRF promoter. (B) Cells transfected with a full-length (CRF-907luc), deleted (CRF-233luc, CRF-248luc, or CRF-278luc), or mutant (CRF-278Mtluc or CRF-248Mtluc) promoter construct, were pre-incubated for 30 min with the medium containing dexamethasone (Dex, 100 nM) or vehicle (C), then incubated for 2 h with the medium containing 10 μM forskolin. Data are presented as relative activity; luciferase activity in response to forskolin alone (C) was set at 100% in all transfected cells; *P < 0.05 (compared with forskolin alone [C] in all transfected cells), +P < 0.05 (compared with Dex in CRF-907luc), ++P < 0.05 (compared with Dex in CRF-907luc and CRF-278luc), and +++P < 0.05 (compared with Dex in CRF-907luc, CRF-278luc, and CRF-248luc). [Reproduced with permission from Kageyama et al. (Citation2008). Copyright © Editrice Kurtis srl.]](/cms/asset/77d30593-a877-46ea-ae39-8f4397f64005/ists_a_536279_f0006_b.gif)
Figure 7. Effects of ICER siRNA on forskolin-induced CRF 5′-promoter activity in 4B cells. Cells were incubated for 24 h in culture medium containing siRNA for either control (siControl) or ICER (siICER). After the incubation, the cells were transfected with CRF promoter constructs, then incubated with 1 μM forskolin alone or vehicle for the durations shown. Forskolin-induced CRF 5′-promoter activity in cells transfected with siControl is shown as open circles, while that in cells transfected with siICER is shown as closed circles; *P < 0.05 (compared with control).
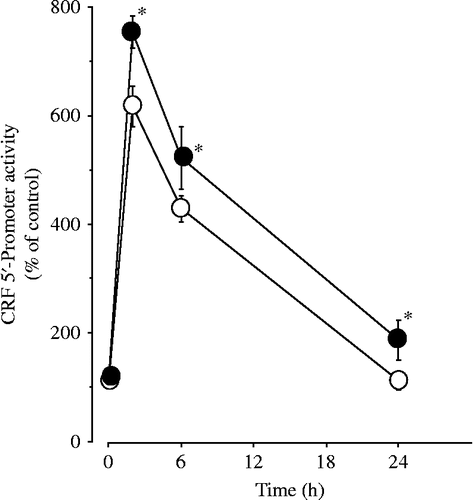
Figure 8. Effects of IL-6 on PACAP-induced CRF 5′-promoter activity in 4B cells. (A) Effects of PACAP on IL-6 production in 4B cells. Cells were incubated for 48 h with medium alone (control), or with medium containing forskolin (F) or PACAP. The titers of IL-6 in the culture supernatants were measured by IL-6 ELISA; **P < 0.005 (compared with control (C)). (B) Effects of anti-IL-6 Ab on PACAP-induced CRF 5′-promoter activity in 4B cells. Cells were pre-incubated with medium containing anti-IL-6 Ab or control IgG for 30 min and then incubated for 2 h with medium containing 10 or 100 nM PACAP or vehicle. Cells treated with control IgG are indicated as C; **P < 0.005 (compared with control (C)), ++P < 0.005 (compared with 100 nM PACAP), and #P < 0.05 (compared with 10 nM PACAP). [Reproduced with permission from Kageyama et al. (Citation2007). Copyright © Society for Endocrinology.]
![Figure 8. Effects of IL-6 on PACAP-induced CRF 5′-promoter activity in 4B cells. (A) Effects of PACAP on IL-6 production in 4B cells. Cells were incubated for 48 h with medium alone (control), or with medium containing forskolin (F) or PACAP. The titers of IL-6 in the culture supernatants were measured by IL-6 ELISA; **P < 0.005 (compared with control (C)). (B) Effects of anti-IL-6 Ab on PACAP-induced CRF 5′-promoter activity in 4B cells. Cells were pre-incubated with medium containing anti-IL-6 Ab or control IgG for 30 min and then incubated for 2 h with medium containing 10 or 100 nM PACAP or vehicle. Cells treated with control IgG are indicated as C; **P < 0.005 (compared with control (C)), ++P < 0.005 (compared with 100 nM PACAP), and #P < 0.05 (compared with 10 nM PACAP). [Reproduced with permission from Kageyama et al. (Citation2007). Copyright © Society for Endocrinology.]](/cms/asset/4b0d0b2f-a573-4348-8d2a-4bf14e3e0941/ists_a_536279_f0008_b.gif)
Figure 9. Effects of SOCS-3 on the regulation of CRF 5′-promoter activity in 4B cells. Cells were incubated for 24 h in culture medium containing siRNA for either control (siControl) or SOCS (siSOCS). After incubation, the cells were transfected with a CRF promoter construct and then incubated with vehicle (A), 10 μM forskolin (Fsk) for 2 h (B) or 100 ng/mL IL-6 for 24 h (C); *P < 0.05 (compared with control [siControl]). [Reproduced with permission from Kageyama et al. (Citation2009a). Copyright © Society for Endocrinology.]
![Figure 9. Effects of SOCS-3 on the regulation of CRF 5′-promoter activity in 4B cells. Cells were incubated for 24 h in culture medium containing siRNA for either control (siControl) or SOCS (siSOCS). After incubation, the cells were transfected with a CRF promoter construct and then incubated with vehicle (A), 10 μM forskolin (Fsk) for 2 h (B) or 100 ng/mL IL-6 for 24 h (C); *P < 0.05 (compared with control [siControl]). [Reproduced with permission from Kageyama et al. (Citation2009a). Copyright © Society for Endocrinology.]](/cms/asset/9295ff38-de2d-491e-8a6e-2138184bc42d/ists_a_536279_f0009_b.gif)
Table I. Microarray analysis in the AP of CRF KO.
Figure 10. Effects of CRF on Pak3 mRNA levels. (A) Time-dependent changes in CRF-induced Pak3 mRNA levels. Cells were incubated with medium containing 100 nM CRF for the times indicated. (B) Effects of a CRF receptor antagonist on CRF-induced Pak3 mRNA levels. Cells were pre-incubated with medium containing 1 μM astressin (AST) or vehicle for 30 min and then incubated for 6 h with medium containing 100 nM CRF or vehicle. (C) Effects of Pak3 on CRF-induced AtT-20 cell viability. The AtT-20 cells were incubated for 24 h in culture medium containing siRNA for either control (siCont) or Pak3 (siPak). After incubation, AtT-20 cells were incubated for 48 h in culture medium containing either CRF (10 or 100 nM) or the vehicle (Veh). Cell viability was measured by WST-1 colorimetric assay; *P < 0.005 (compared with control (0 h or C)). [Reproduced with permission from Kageyama et al. (Citation2009b). Copyright © Elsevier.]
![Figure 10. Effects of CRF on Pak3 mRNA levels. (A) Time-dependent changes in CRF-induced Pak3 mRNA levels. Cells were incubated with medium containing 100 nM CRF for the times indicated. (B) Effects of a CRF receptor antagonist on CRF-induced Pak3 mRNA levels. Cells were pre-incubated with medium containing 1 μM astressin (AST) or vehicle for 30 min and then incubated for 6 h with medium containing 100 nM CRF or vehicle. (C) Effects of Pak3 on CRF-induced AtT-20 cell viability. The AtT-20 cells were incubated for 24 h in culture medium containing siRNA for either control (siCont) or Pak3 (siPak). After incubation, AtT-20 cells were incubated for 48 h in culture medium containing either CRF (10 or 100 nM) or the vehicle (Veh). Cell viability was measured by WST-1 colorimetric assay; *P < 0.005 (compared with control (0 h or C)). [Reproduced with permission from Kageyama et al. (Citation2009b). Copyright © Elsevier.]](/cms/asset/e0c7bef3-ef4c-4d60-9729-246fe07cae40/ists_a_536279_f0010_b.gif)
Figure 11. A schematic model of modulation of the HPA axis in Nelson's syndrome. Nelson's syndrome results from the development of an aggressive ACTH-secreting pituitary adenoma in patients who have undergone bilateral adrenalectomy for Cushing's disease.
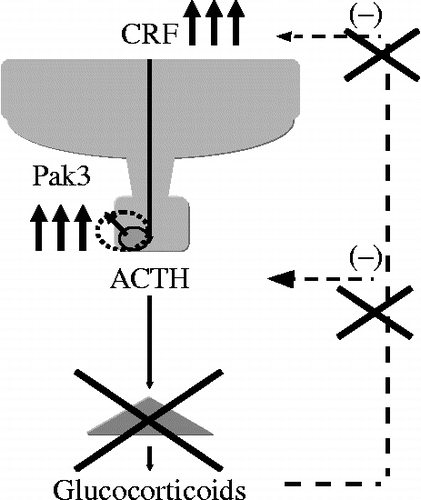
Figure 12. Processing of POMC in the AP of CRF KO. (A) Processing of POMC to the POMC-related peptides in the AP. POMC-related peptides are obtained by PC-1 and PC-2 in the AP. In the processing of POMC to NT, JP, ACTH, and β-LPH are mainly produced by the several steps of PC-1, whereas γ-LPH and β-EP are a little produced by PC-2. As β-EP Ab has cross-reactivities with POMC and β-LPH (shown as a dark block square), ir β-EP contains POMC, β-LPH and β-EP, which were previously determined. NT, N-terminal peptide; JP, joining peptide; MSH, melanocyte stimulating hormone; CLIP, corticotropin-like intermediate lobe peptide; LPH, lipotropic hormone; EP, endorphin; PC, prohormone convertase. (B) Representative elution profiles of ir β-EP contents extracted from the AP of WT and CRF KO by Sephadex G-50 gel filtration chromatography. The gel filtration analyses revealed that a higher molecular weight form of ir β-EP, putative POMC, was increased in CRF KO, but the β-EP peak level was small and similar between the two groups. Vo, void volume; LPH, lipotropic hormone; EP, endorphin. (C) POMC/β-LPH ratio in the AP of WT and CRF KO by Sephadex G-50 gel filtration chromatography. POMC/β-LPH ratio of anterior pituitary from individual mice are shown as the mean ± SEM (n = 4); *P < 0.001 (compared with WT). [Reproduced with permission from Fukuda et al. (Citation2004). Copyright © Elsevier.]
![Figure 12. Processing of POMC in the AP of CRF KO. (A) Processing of POMC to the POMC-related peptides in the AP. POMC-related peptides are obtained by PC-1 and PC-2 in the AP. In the processing of POMC to NT, JP, ACTH, and β-LPH are mainly produced by the several steps of PC-1, whereas γ-LPH and β-EP are a little produced by PC-2. As β-EP Ab has cross-reactivities with POMC and β-LPH (shown as a dark block square), ir β-EP contains POMC, β-LPH and β-EP, which were previously determined. NT, N-terminal peptide; JP, joining peptide; MSH, melanocyte stimulating hormone; CLIP, corticotropin-like intermediate lobe peptide; LPH, lipotropic hormone; EP, endorphin; PC, prohormone convertase. (B) Representative elution profiles of ir β-EP contents extracted from the AP of WT and CRF KO by Sephadex G-50 gel filtration chromatography. The gel filtration analyses revealed that a higher molecular weight form of ir β-EP, putative POMC, was increased in CRF KO, but the β-EP peak level was small and similar between the two groups. Vo, void volume; LPH, lipotropic hormone; EP, endorphin. (C) POMC/β-LPH ratio in the AP of WT and CRF KO by Sephadex G-50 gel filtration chromatography. POMC/β-LPH ratio of anterior pituitary from individual mice are shown as the mean ± SEM (n = 4); *P < 0.001 (compared with WT). [Reproduced with permission from Fukuda et al. (Citation2004). Copyright © Elsevier.]](/cms/asset/617506c0-ed87-4c82-b494-a99677356ca4/ists_a_536279_f0012_b.gif)
Figure 13. Effects of CRF on PC-1 mRNA levels. (A) Time-dependent changes in CRF-induced PC-1 mRNA levels. Cells were incubated with the medium containing 100 nM CRF for the times indicated. (B) Effects of a CRF-receptor antagonist on CRF-induced PC-1 mRNA levels. Cells were pre-incubated with the medium containing 1 μM AST or vehicle for 30 min and then incubated for 6 h with the medium containing 100 nM CRF or vehicle; *P < 0.005 (compared with control (0 h or C)). [Reproduced with permission from Kageyama et al. (Citation2009b). Copyright © Elsevier.]
![Figure 13. Effects of CRF on PC-1 mRNA levels. (A) Time-dependent changes in CRF-induced PC-1 mRNA levels. Cells were incubated with the medium containing 100 nM CRF for the times indicated. (B) Effects of a CRF-receptor antagonist on CRF-induced PC-1 mRNA levels. Cells were pre-incubated with the medium containing 1 μM AST or vehicle for 30 min and then incubated for 6 h with the medium containing 100 nM CRF or vehicle; *P < 0.005 (compared with control (0 h or C)). [Reproduced with permission from Kageyama et al. (Citation2009b). Copyright © Elsevier.]](/cms/asset/25c0afb4-3134-4c0b-bd74-b390236a9126/ists_a_536279_f0013_b.gif)