Figures & data
Figure 2. Proposed scheme for naphthalene metabolism and reactive metabolites (adapted from CitationATSDR 2005). CYP450 = Cytochrome P450 Enzyme(s); GSH = Reduced Glutathione; SG = Glutathione.
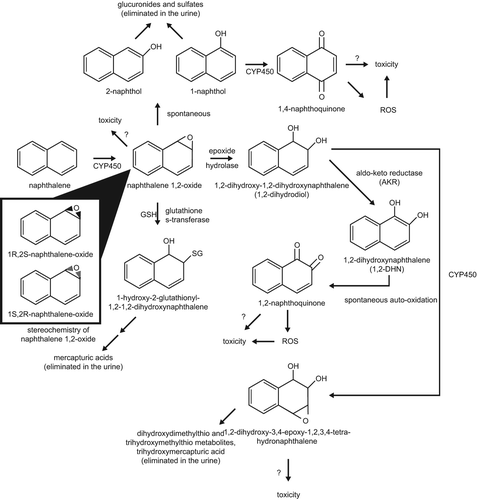
Figure 3. Amount of naphthalene metabolized in rat and human dorsal olfactory, ventral respiratory, lung, and liver tissue per inhaled dose in accordance with the hybrid CFD–PBPK model for naphthalene (CitationCampbell et al. 2014).
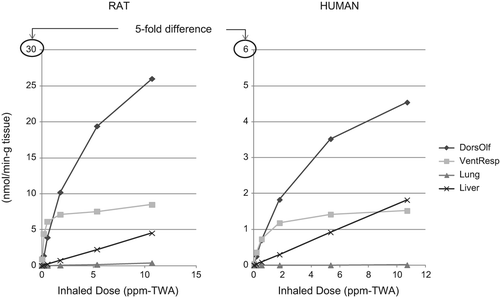
Table 1. Human AKRs and activity toward naphthalene metabolites.
Table 2. Key Events in the proposed modes of action for naphthalene-induced carcinogenesis.
Table 3. Comparative Reasoning for Accounts of Naphthalene Carcinogenesis in Rodents and Human Relevance.
Table 4. Points of departure for lesions in naphthalene-exposed rats from NTP (CitationNTP 2000) and CitationDodd et al. (2012)*.
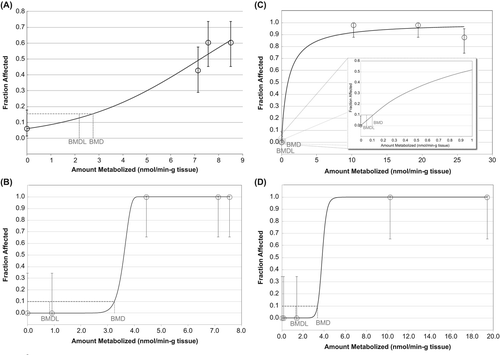
Figure 4. Two-year (CitationNTP 2000) vs 90-day (CitationDodd et al. 2012) metabolized dose–response in male and female rats following naphthalene exposure via inhalation (BMR of 10% extra risk for the 0.95 lower confidence limit on the BMD). Figures show the best-fit curve. See supplemental material for all modeling results. (A) Male rat respiratory epithelial hyperplasia dose–response from 2-year study (Logistic); (B) male rat respiratory epithelial hyperplasia dose–response from 90-day study (Weibull); (C) female rat olfactory epithelial hyperplasia dose–response from 2-year study (Log-Logistic with NO FIT); (D) female olfactory epithelial hyperplasia dose–response from 90-day study (Log-Logistic).