Figures & data
Table I. Characteristics of controlled ESA trials conducted in patients with lymphoproliferative malignancies that reported mortality data.
Figure 1. Study-level meta-analysis of mortality in randomized controlled trials of patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls, including odds ratio for death using a random-effects model and results of a risk difference analysis. *The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. reported that the hazard ratio for overall survival was 1.02 (95% CI, 0.55–2.05). Because the Aapro report did not include the numbers or percentages of patients who died, its data for the Österborg 1996 study could not be included in this meta-analysis.
![Figure 1. Study-level meta-analysis of mortality in randomized controlled trials of patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls, including odds ratio for death using a random-effects model and results of a risk difference analysis. *The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. reported that the hazard ratio for overall survival was 1.02 (95% CI, 0.55–2.05). Because the Aapro report did not include the numbers or percentages of patients who died, its data for the Österborg 1996 study could not be included in this meta-analysis.](/cms/asset/f05faf5f-3aca-42b2-814f-df97032ba49c/ilal_a_684347_f0001_b.gif)
Figure 2. Study-level meta-analysis of disease progression in patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls; odds ratio for disease progression using a random-effects model. *In the Österborg 1996 and 2005 studies [Citation7,Citation27], disease progression was based on tumor response assessment. †The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. [Citation35] reported that the hazard ratio for disease progression was 1.43 (95% CI, 0.72–2.86). Because the Aapro report did not include the numbers or percentages of patients with disease progression, its data for the Österborg 1996 study could not be included in this meta-analysis.
![Figure 2. Study-level meta-analysis of disease progression in patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls; odds ratio for disease progression using a random-effects model. *In the Österborg 1996 and 2005 studies [Citation7,Citation27], disease progression was based on tumor response assessment. †The Österborg 1996 study was included in a meta-analysis of epoetin beta studies reported by Aapro et al. in 2006 [Citation35], with 4 weeks of follow-up beyond the period reported in the original 1996 publication [Citation7]. Aapro et al. [Citation35] reported that the hazard ratio for disease progression was 1.43 (95% CI, 0.72–2.86). Because the Aapro report did not include the numbers or percentages of patients with disease progression, its data for the Österborg 1996 study could not be included in this meta-analysis.](/cms/asset/7ed8d6a1-b71e-404d-a709-1f60979e5734/ilal_a_684347_f0002_b.gif)
Figure 3. Proportion of patients (%) who received transfusions in studies of patients with lymphoproliferative malignancies receiving erythropoiesis-stimulating agents (ESAs) or controls in randomized controlled trials. n represents the number of patients for whom transfusion data were available in each study.
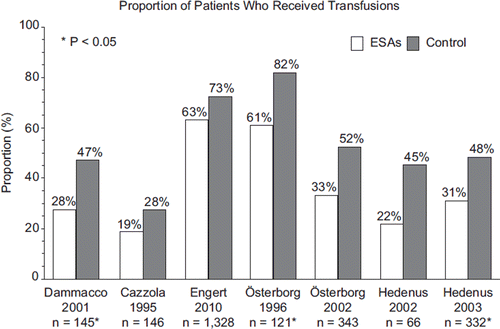
Table II. Thromboembolic or cardiovascular events reported* in studies included in meta-analysis of patients with lymphoproliferative malignancies.