Figures & data
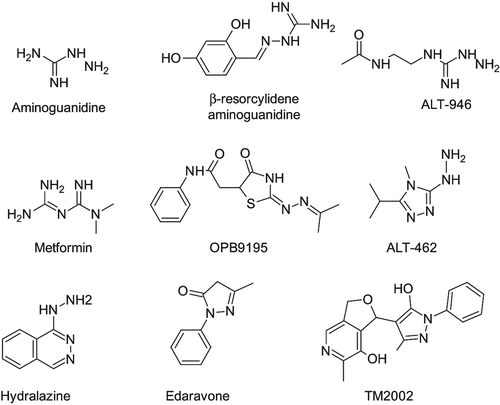
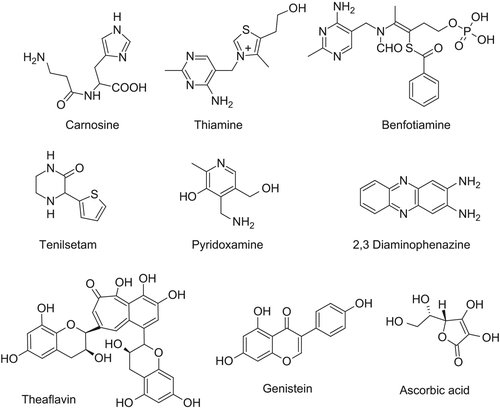
Figure 3. Relevant xenobiotics acting as antioxidants (first row) and metal chelators (second and third rows).
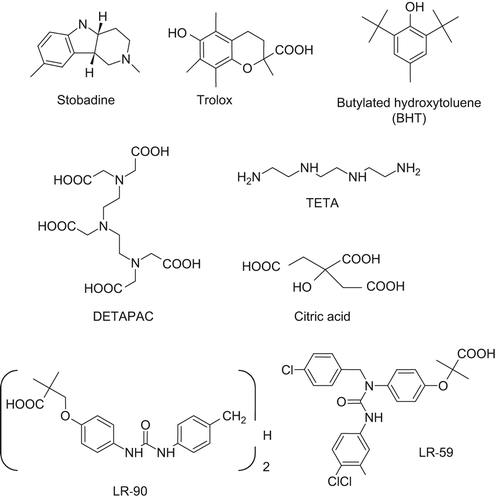
Figure 8. Structure of AGEs breakers (lower panel) and proposed reaction mechanism of thiazolium-derived AGEs breakers (upper panel). The simplified reaction mechanism is referred to the dinucleophilic attack of the thiazolium ring toward the dicarbonyl AGE cross-link followed by internal rearrangement and hydrolysis.
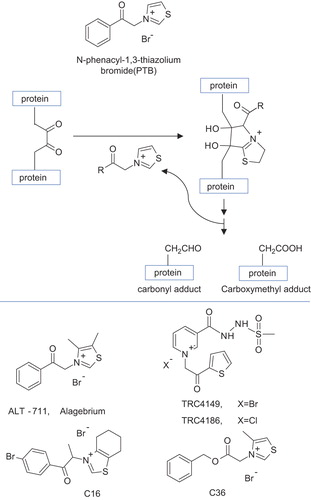
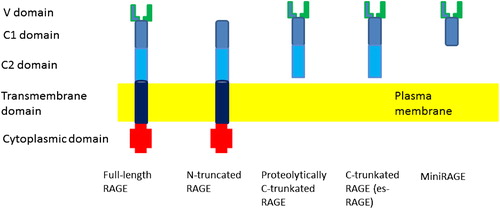
Figure 9. The structure of full-length RAGE and its variants. The V-type domain is critical for binding of RAGE–ligand axis. Deletion of this domain results in an N-truncated form that does not bind ligands. The C-truncated, circulating soluble RAGE contains only the extracellular domain of the receptor. It may be a result of alternate endogenous splicing (esRAGE) or proteolytic cleavage.