Figures & data
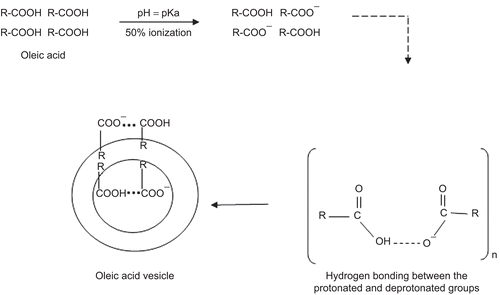
Table 1. Size and entrapment efficiency of the prepared oleic acid vesicles.
Figure 1. Vesicles morphology of optimized oleic acid vesicles by (A) optical microscopy (400× magnification) and (B) TEM at 80 kV and 50,000×.
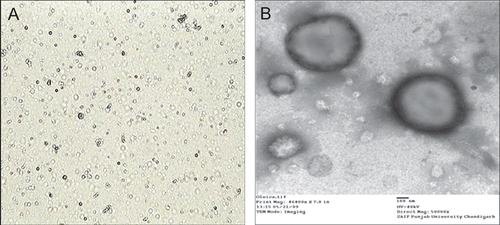
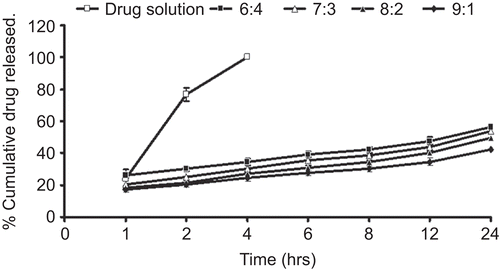
Table 2. Order of drug release of various formulations determined by the regression coefficients.
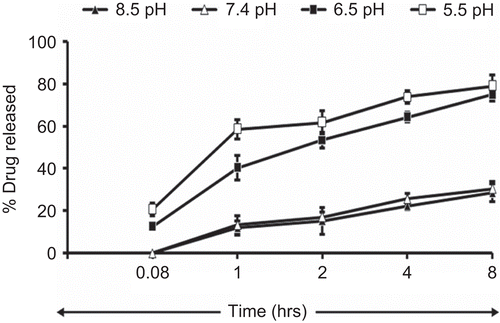
Figure 4. Photomicrograph of oleic acid vesicles dispersion incubated at different pH (400× magnification).
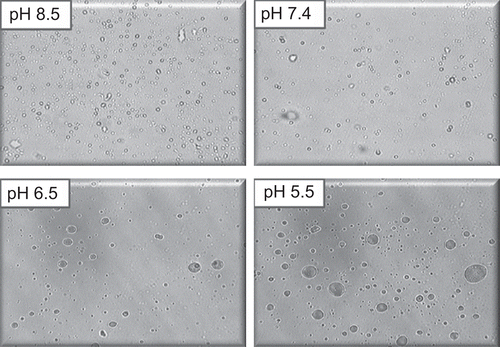
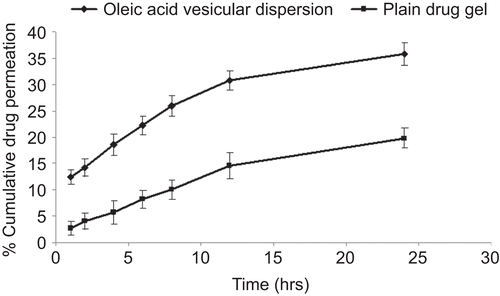
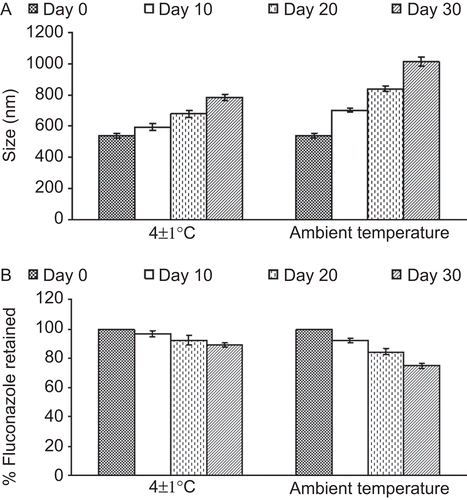
Table 3. Skin permeation and retention of fluconazole from various formulations.
Figure 6. Confocal microscopic image of skin showing penetration behavior of oleic acid vesicles along the thickness of skin in vivo. It can be observed that the fluorescence intensity is detectable up to a thickness of 55–60 μm. In the upper part of the epidermis some intact vesicles were also observed.
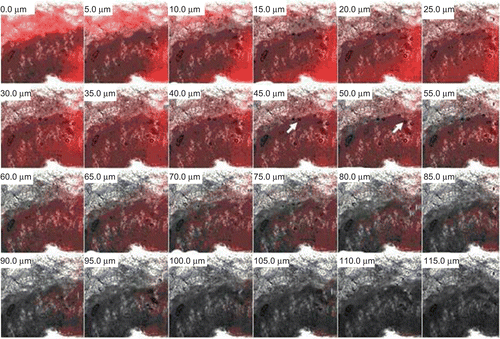
Table 4. Skin irritation response scores to oleic acid vesicles (Er and Ed are erythemal and edemal irritation index, respectively).
Figure 7. Percentage drug unabsorbed with respect to time after application of oleic acid vesicle dispersion and plain drug gel on skin in vivo.
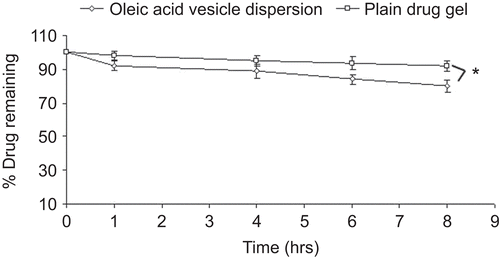
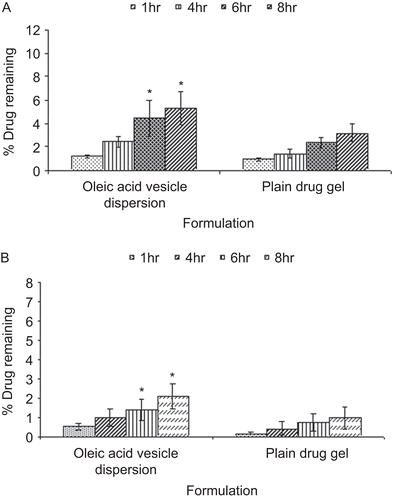
Figure 8. In vivo drug localization in (A) SC, and (B) viable layers of the skin for oleic acid vesicle dipsersion and plain drug gel.