Figures & data
Figure 1. Diffusion pattern of Gd-DTPA after injection into different brain regions. (A) Distribution of Gd-DTPA after IC injection into the caudate putamen over a period of 6 hours. The distribution area was outlined, and symbols of ‘*’, ‘#’ and ‘†’ presented the posterior caudate nucleus, the frontotemporal cortex and the anterior thalamus individually. (B) Distribution of Gd-DTPA 2 hours after ICV injection. To avoid the influence on the distribution by the reflux from the needle track to the cortex, Gd-DTPA was injected into the contralateral ventricle. Gd-DTPA could hardly diffuse to the parenchyma from the contralateral ventricle. Most of the MCA territory (‘*’ and ‘#’) except for the thalamus (‘†’) was excluded from the distribution area of Gd-DTPA. The subarachnoid space (‘→’) was significantly enhanced by Gd-DTPA. (C) Gd-DTPA diffused in a manner of anisotropy and spread along the white fiber after IC injection into the external capsule. (D) Limited deposition of Gd-DTPA was found 1 hour after IC injection into the thalamus.
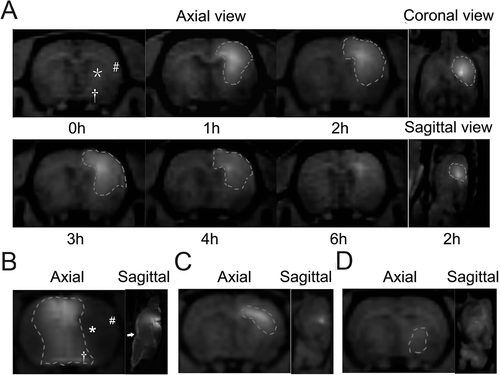
Figure 2. Pharmacokinetic modeling of citicoline by MRI tracer method. (A) The quantitative linear curve of MRI signal enhancement (ΔSI) versus Gd-DTPA concentration (C) in rat brain tissues over the range of 0~0.1mmol/L. (B) Sketch map of the potential infarct regions after pMCAO procedure. (C) Distribution of Gd-DTPA concentrations in different brain regions over a period of 6 hours. (D) TTC staining of ischemic lesions in groups treated with different dose of citicoline. (E) Comparison of the infarct volume ratio among groups treated with different dose of citicoline. Data were mean±SD, n=5~8. *P < 0.05, **P < 0.01.
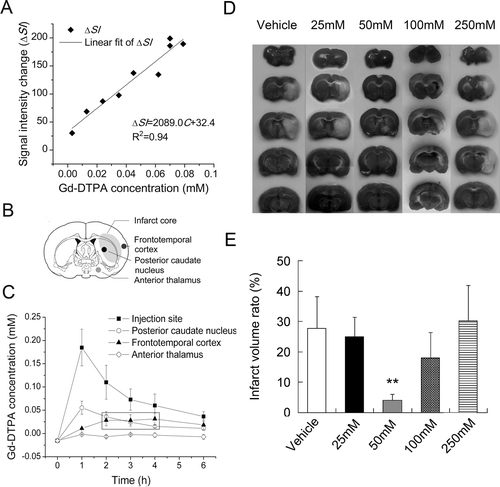
Figure 3. Stereotactic delivery of citicoline reduced the infarct volume ratio and the neurological deficit. (A) TTC staining and MRI of ischemic lesions in groups treated with different drug delivery. Infarct tissues were not stained by TTC. Typical manifestations of infarct tissues were decreased signal intensity on T1W images and increased signal intensity on T2W images. The characteristics lesions were observed to be involving both the cortex (arrow with a round head) and the subcortical regions (arrows with a square head and a triangular head). (B) Comparison of the infarct volume ratio among groups. Data were mean±SD, n=8. *P < 0.05, **P < 0.01. (C) Comparison of the neurological deficit score among groups. Data were mean±SD, n=8. *P < 0.05, **P < 0.01. (D) H&E staining of neurons. Normal neurons (‘→’) with an intact mesenchyme (‘*’) was shown in sham group. In vehicle group and IP group, severe eosinophilic degeneration (‘→’) was observed in most neurons with a collapsed mesenchyme (‘*’). Lymphocytes were found around necrotic cells (‘▸’). In IC group, a milder injury occurred in the cortex and the caudate nucleus except for the thalamus.
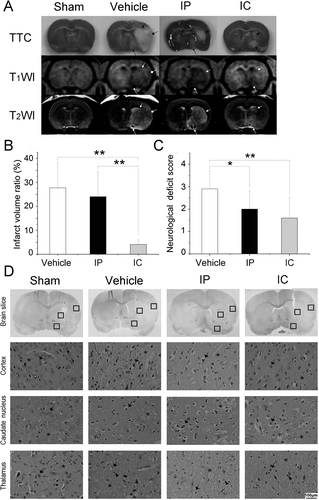
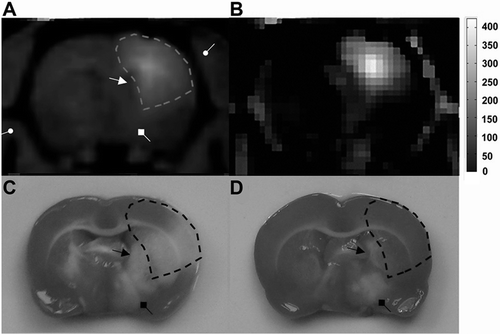
Figure 4. Potentially protected regions predicted by MRI tracer method. (A) Axial image of Gd-DTPA enhanced T1WI provided the anatomical information of brain tissues neighboring the ventricle (arrow with a triangular head), the thalamus (arrow with a square head) and the skull (arrow with a round head). (B) Pseudo-color image of MR signal enhancement 2 hours after IC injection of Gd-DTPA. (C) Characteristic ischemic lesions involved both the cortex and subcortical regions. (D) Non-deposition areas of Gd-DTPA matched exactly with the poorly protected regions in pMCAO experiment by stereotactic delivery of citicoline.