Figures & data
Table 1. Liposome properties (pH: 7.4, Mean ± PDI, n = 5 in each series).
Figure 1. Liposome morphology by microscope (450X). The shape of liposome was observed at a magnification of 450X through the inverted microscope (TE2000-U, Nikon, Japan).
Figure 2. Ethinylestradiol retention of various vesicle formulations. The various formulations were in aqueous buffer, pH = 7.4, stored at 4°C for 28 days. Ethinylestradiol was encapsulated and drug retention was measured on the basis of un-entrapped ethinylestradiol recovered in the suspension following centrifugation at 20 000g for 30 min. Results represent percentage retention of initially incorporated ethinylestradiol and are expressed as the means of five experiments ± SD.
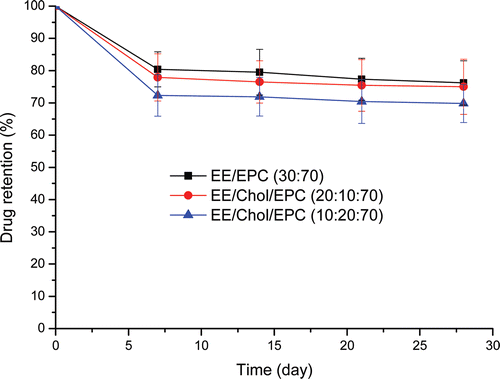
Figure 3. Esthinylestradiol release from liposomes. The cumulative release profile was under physiological conditions from formulations in aqueous buffer, pH = 7.4, at 37°C. Esthinylestradiol was encapsulated and drug release was measured on the basis of un-entrapped esthinylestradiolrecovered in the suspension following centrifugation at 20 000g for 30 min at each time-point. Results represent percentage cumulative release of initially incorporated esthinylestradiol and were expressed as the means of four experiments ± SD.
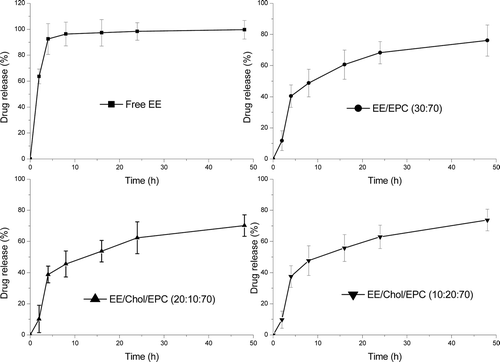
Table 2. Kinetic values of release esthinylestradiol from different liposomal formulations with and without cholesterol containing using the correlation coefficient parameter.
Figure 4. The body weight changes during the experiment time (vs. ovx: *p<0.05; vs. sham: #p<0.05). The body weights of rats were measured using electronic balance every 30 days during the 3 months post-surgery and every 15 days during the following 1 month therapy. The changes of body weight were reflected using the data of ΔG, which was calculated by the difference between the G0d and G120d, that was ΔG = G120d - G0d. Results represent the rats living condition with the drug administration during the whole experiment period and were expressed as the means of eight animals ± SD.
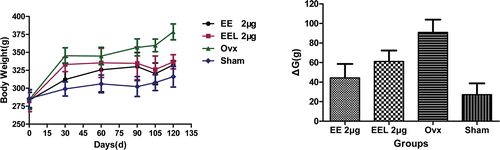
Figure 5. BMD value and length of rats’ right femur (vs. ovx: *p<0.05). The bone mineral density (BMD, mg/cm2) was measured by PRODIGY Direct Digital DEXA (dual-energy X-ray absorptiometry) Bone Densitometry (GE Prodigy, USA) at different time internal. The length of the right femur was measured with a micrometer. Bone mass was measured by electronic balance (MP6001, quantity sensitive: 0.01g, China). Results were expressed as the means of eight animals ± SD.
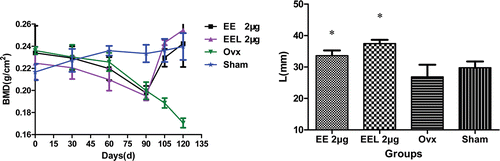
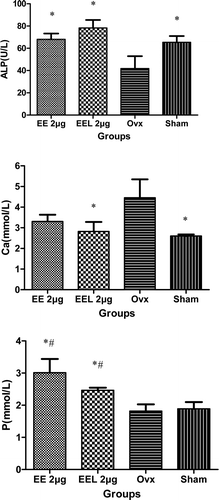
Figure 6. The serum alkaline phosphatase (ALP), calcium (Ca), and phosphours (P) contents in serum (vs. ovx: *p<0.05▪vs. sham: #p<0.05). ALP, Ca and P contents in serum were detected using fully-automatic biochemical meter (7080, HITACHI, Japan). Results represent the bone formation after esthinylestradiol supplementary therapy and were expressed as the means of eight experiments ± SD.