Figures & data
Table 1. Composition of liposomes and their respective particle size with zeta potential values.
Figure 1. (A) Transmission electron micrograph of pegylated liposomes (C1) of PLB. (B) Encapsulation efficiency (%) of PLB. F1-F5 represents conventional, whereas C1 represents long circulating liposomes. (C) Effect of cholesterol on in vitro release profile of conventional (CH1-CH4) and long circulating liposomes (C1) in PBS at 37 °C (n = 3).
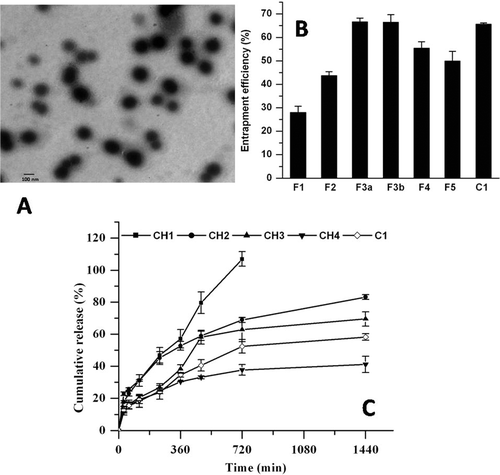
Table 2. Effect of cholesterol on physicochemical characteristics of liposomes.
Figure 2. Physical stability of conventional (F3b) and long circulating liposomes (C1) in fetal bovine serum as a function of particle size up to 24 h incubation. Significant values: **p < 0.01 in comparison to particle size at initial time point.
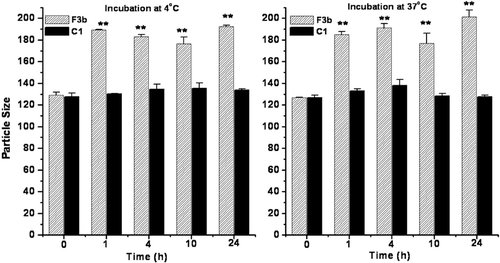
Table 3. Pharmacokinetic parameters of PLB, free and encapsulated as conventional and pegylated liposomes, after intravenous bolus administration at a dose of 6 mg/kg to tumor bearing mice (n = 4).
Figure 3. Plasma concentration of PLB obtained from mice after intravenous administration of free PLB, conventional (F3b) and pegylated liposomes (C1) at a dose of 6 mg/kg. The data represents mean ± SD of four animals.
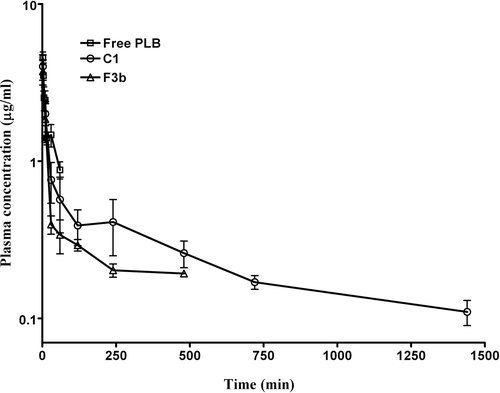
Table 4. Tumor growth parameters for free PLB and liposomal formulations (n = 8).
Figure 4. Effect of free PLB and formulated liposomes as conventional or pegylated (A) on tumor growth inhibition and (B) survival of C57BL/6J mice inoculated with B16F1 melanoma cells. Significant levels - ** p < 0.001 compared with vehicle treated group; a p < 0.01, b p < 0.001 compared to free plumbagin treated group. When tumor volume became about 100 ± 10 mm3, free PLB, conventional liposomes (F3b) or pegylated liposomes was intravenously injected (containing 2 mg/kg PLB each time on days 1, 3, 5, 7 and 10 (total dose of 10 mg/kg)). Control animals were injected with the vehicle (PBS containing 25% PEG-200). Each data represents the mean ± SD (n = 8).
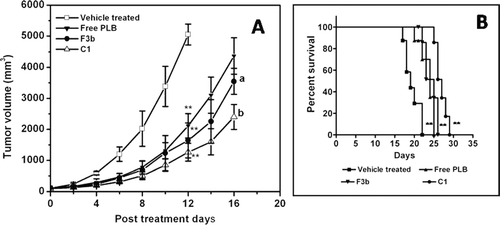
Table 5. Hematological changes after intravenous administration of free PLB and pegylated liposomes (C1) for 5 consecutive days.
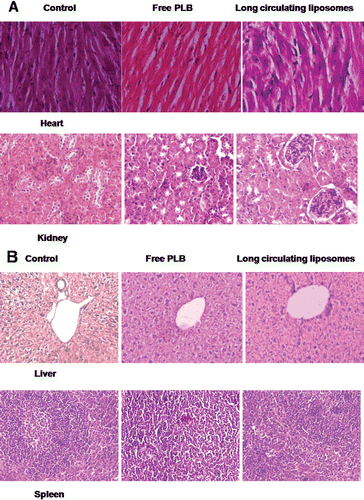
Figure 5. Toxicity assessment of free PLB and long circulating liposomes (C1) in C57BL/6J mice. The histopathologic examination of tissue sections of (A) heart and kidney, (B) liver and spleen was made after mice were sacrificed and staining with H&E stain. All the treatment groups showed no signs of toxic pathological change; magnification 400X; 100X for liver.