Figures & data
Table 1. A 23 full factorial design of the prepared PCL single-coat celecoxib-loaded microparticles.
Figure 1. DSC thermograms of celecoxib, PCL, celecoxib: PCL physical mixture and formula F2 (A) and celecoxib, PCL, Eudragit® S100, celecoxib: PCL: Eudragit® S100 physical mixture and formula F10 (B).
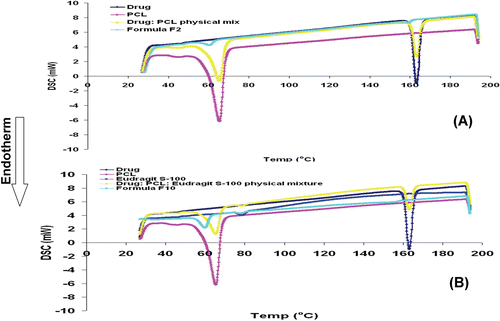
Figure 3. A. Optical micrographs of PCL single-coat microparticles, formula F2 (A), and double-coat microparticles, formula F10 (B). B. SEM micrographs of PCL single-coat microparticles, formula F2 (C), and double-coat microparticles, formula F10 (D). Scale bar represents 300 μm, X160.
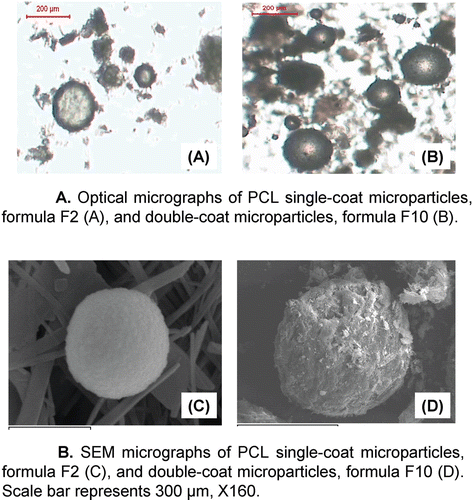
Table 2. The particle size and the entrapment efficiency % of the prepared celecoxib-loaded microparticles, mean ± S.D., n = 3.
Figure 4. Interaction bar plot representing the effect of PCL M.wt. (low and high), drug: PCL ratio (1:0.5 and 1: 1) and span® 80 concentration (1% and 3%, w/v) on the entrapment efficiency %, mean ± S.D.
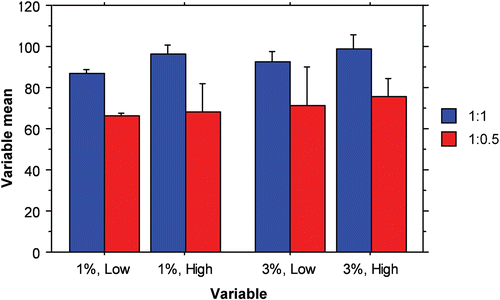
Figure 5. The influence of PCL M.wt. and drug: PCL ratio on in vitro drug release from PCL single-coat microparticles prepared using Span® 80 (1%, w/v) at 37 ± 0.5 °C (mean ± S.D., n = 3).
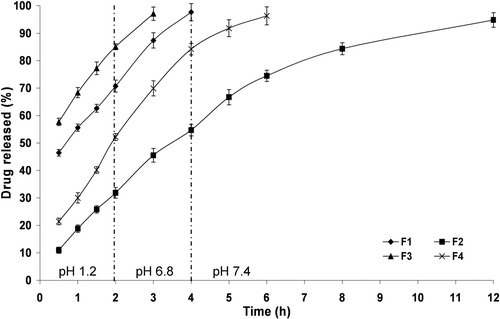
Figure 6. The influence of PCL M.wt. and drug: PCL ratio on in vitro drug release from PCL single-coat microparticles prepared using Span® 80 (3%, w/v) at 37 ± 0.5 °C (mean ± S.D., n = 3).
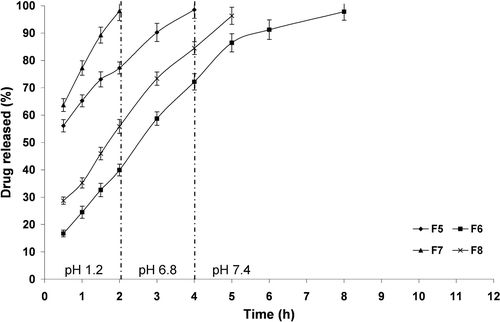
Table 3. Mathematical modeling and release kinetics of celecoxib from the investigated microparticles.
Figure 7. Interaction bar plots representing the effect of PCL M.wt. (low and high), drug: PCL ratio (1:0.5 and 1: 1) and Span® 80 concentration (1% and 3%, w/v) on Q2h (A) and Q4h (B), mean ± S.D.
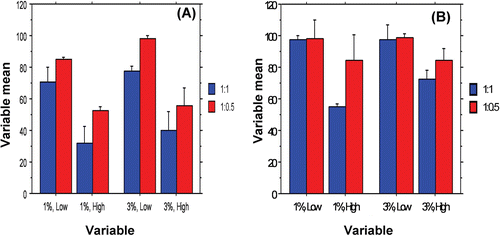
Figure 8. In vitro drug release from PCL single-coat microparticles (F2), Eudragit® S100 single-coat microparticles (F9) and double-coat microparticles (F10) at 37 ± 0.5 °C (mean ± S.D., n = 3).
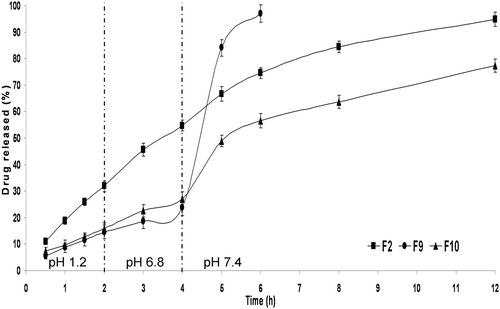
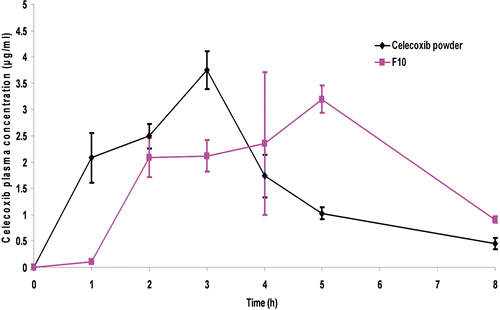
Table 4. Pharmacokinetic parameters of celecoxib following oral administration of aqueous suspensions of the best achieved microparticles (Formula F10) and celecoxib powder to rats (mean ± SD, n = 6).