Figures & data
Table 1. Preparation of SNEDDS as per the experimental design.
Figure 3. Plot between mean percent carvedilol release for all 13 formulations prepared as per 2-factor central composite design where SCD 5 represents the mean of five formulations. The insert shows the release behavior of formulations in 20 min and mean rate of release vs midpoint of time interval.
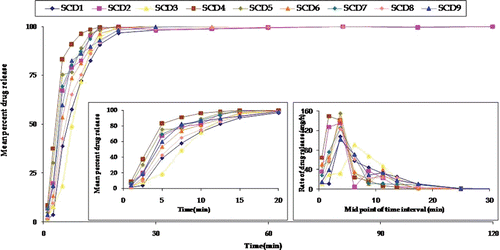
Figure 4. Response surface plot and the corresponding contour plot showing the influence of Cremophor and Transcutol on Q5min for self-emulsifying formulations of carvedilol.
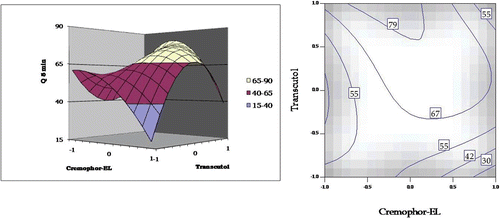
Figure 5. Response surface plot and the corresponding contour plot showing the influence of Cremophor and Transcutol on mean dissolution time (MDT) values for self-emulsifying formulations of carvedilol.
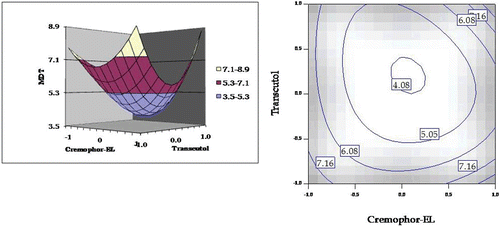
Figure 6. Response surface plot and the corresponding contour plot showing the influence of Cremophor and Transcutol on emulsion droplet size (surface length diameter) for self-emulsifying formulations of carvedilol.
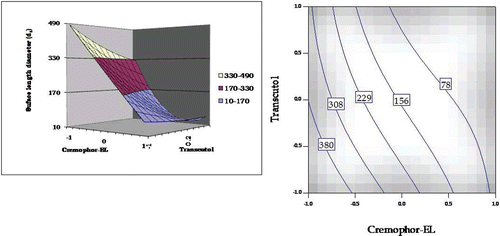
Figure 7. Response surface plot and the corresponding contour plot showing the influence of Cremophor and Transcutol on emulsification time for self-emulsifying formulations of carvedilol.
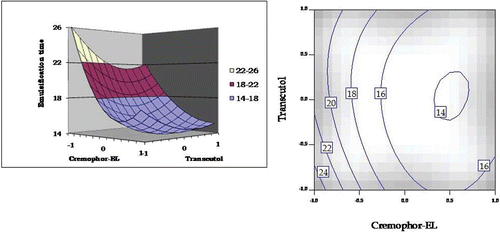
Figure 8. Linear plot and residual plot between the observed and predicted values of (a) Q5min, (b) MDT, (c) droplet size, and (d) emulsification time.
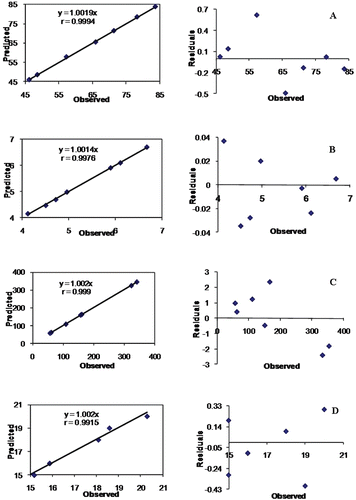
Figure 9. Plot showing mean percent carvedilol release of pure drug, marketed formulation, and two optimized formulations. The insert shows the mean rate of release vs midpoint of time interval.
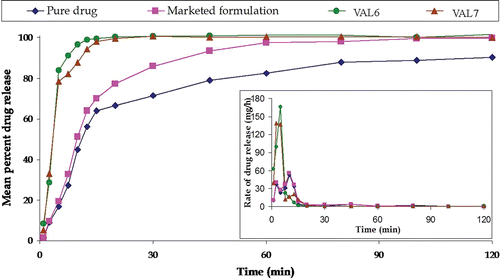
Figure 10. Plot depicting percent increase in permeability parameters (intrinsic permeability, effective permeability, and wall permeability) in marketed formulation and optimized formulations, VAL6 and VAL7, vis-à-vis pure drug using an ex-vivo SPIP technique.
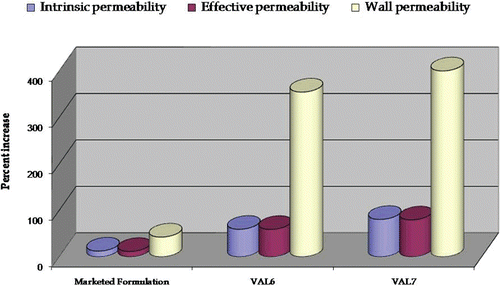
Figure 11. Plot depicting percent increase in absorption parameters (bioavailable fraction and absorption number) in marketed formulation and optimized formulations, VAL6 and VAL7, vis-à-vis pure drug using an ex-vivo SPIP technique.
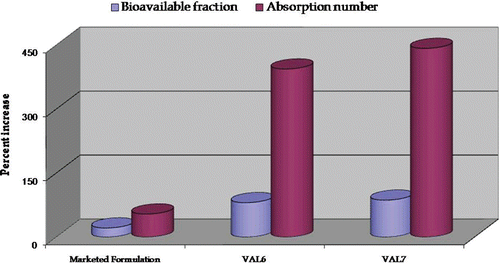
Table 2. Effect on the parameters during the accelerated stability studies kept at 25°C ± 2°C/60% RH ± 5% RH and 40°C ± 2°C/75% RH ± 5%.