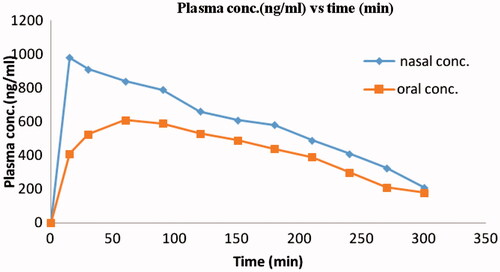
Figures & data
Table 1. Formula for different batches of ondansetron hydrochloride-loaded CPG microspheres.
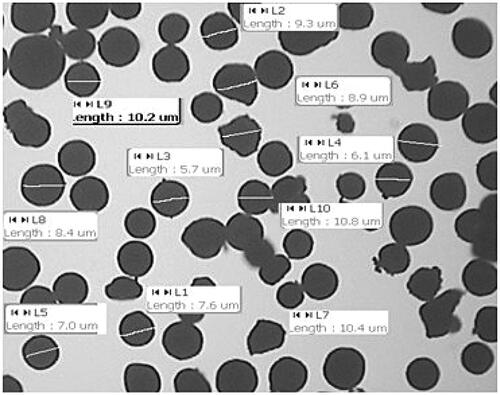
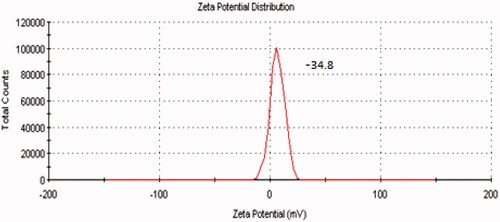
Table 2. Characteristics of prepared ondansetron hydrochloride-loaded microspheres.
Figure 4. DSC spectra of ondansetron hydrochloride (OND), CPG and ondansetron hydrochloride-loaded microspheres (OCP).
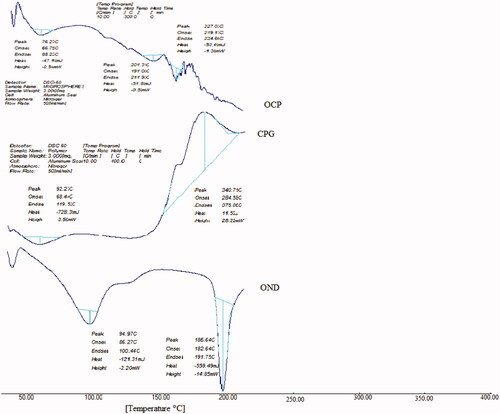
Figure 5. X-ray diffractogram of ondansetron hydrochloride (OND), CPG and ondansetron hydrochloride-loaded microspheres (OCP).
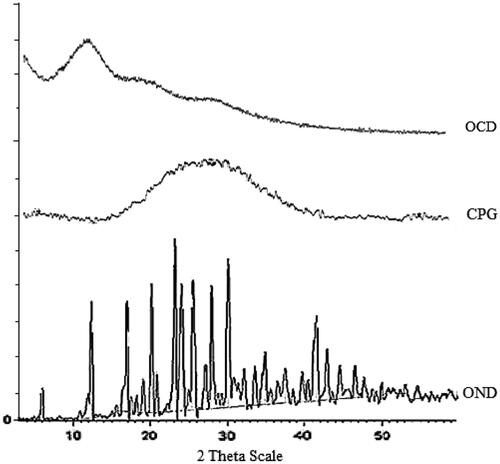
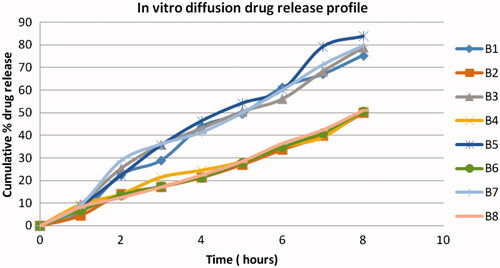
Table 3. In-vitro release kinetic parameter of ondansetron hydrochloride-loaded CPG from microspheres.
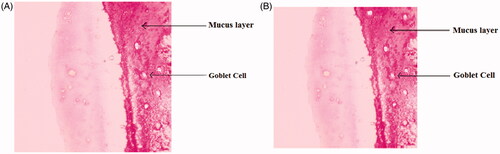
Figure 7. Light photomicrograph of nasal mucosa, untreated mucosa (A) and microspheres treated mucosa (B).