Figures & data
Table 1. The composition and physical properties of the compression-coated tablets.
Figure 1. Release profile of indomethacin from compression-coated tablet prepared with constant pectin amount and varying amounts of calcium chloride. The hardness (crushing strength) of the tablets was around 7.0 kg. Release test was initially done in 900 mL of 0.1 M hydrochloride solution and thereafter was transferred at 2 h to 900 mL pH 6.8 PBS. Both the release media was thermostatically maintained at 37 ± 0.5 °C. Experiments were done in triplicate.
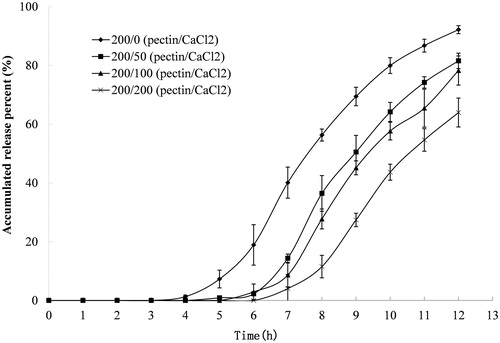
Figure 2. Release profile of indomethacin from compression-coated tablet prepared with different coating weights and a fixed pectin/calcium chloride ratio of 1/1. The hardness (crushing strength) of the tablets was around 7.0 kg. Release test was initially done in 900 mL of 0.1 M hydrochloride solution and was thereafter transferred at 2 h to 900 mL pH 6.8 PBS. Both the release media was thermostatically maintained at 37 ± 0.5 °C. Experiments were done in triplicate.
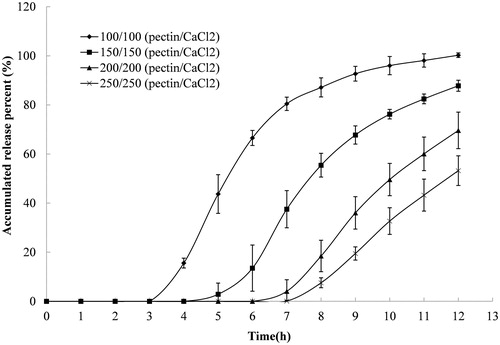
Figure 3. Release profiles of indomethacin from compression-coated tablet at pectin/calcium chloride coating weight of 250/250 (mg/mg) in pH 6.8 PBS containing different concentrations of rat cecal contents, thermostatically maintained at 37 ± 0.5 °C. The hardness (crushing strength) of the tablets was around 7.0 kg. Experiments were done in triplicate.
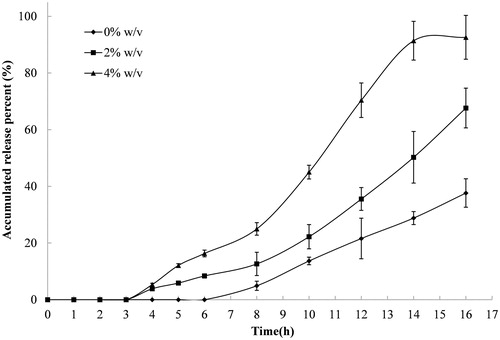
Figure 4. Release profiles of indomethacin from compression-coated tablet at pectin/calcium chloride coating weights of 200/200 and 250/250 (mg/mg). The hardness (crushing strength) of the tablets was around 7.0 kg. The release test was initially done in 900 mL of 0.1 Mhydrochloride solution and was thereafter transferred at 2 h to 900 mL PBS with and without Pectinex Ultra SP-L added to the dissolution media 5 h after the initiation of the release. Experiments were done in triplicate.
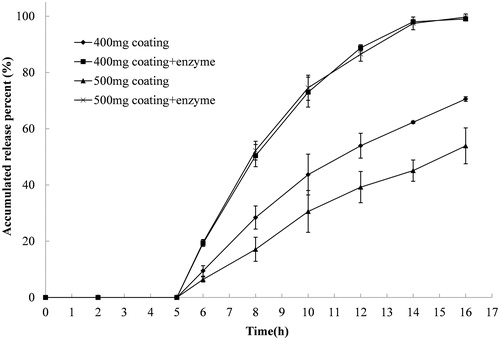
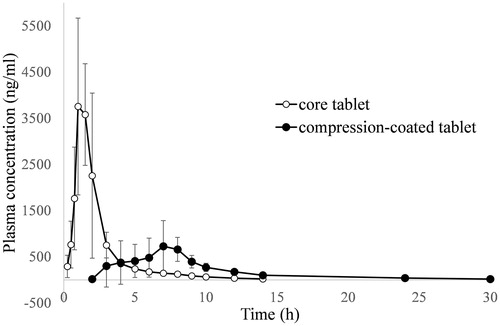
Figure 5. Plasma indomethacin concentration versus time plot after a single oral dose of core tablet and compression-coated tablet at Pectin/calcium chloride coating weight of 250/250 (n = 6).