Figures & data
Table 1. Independent variables and their matching levels for nanosuspension preparation.
Table 2. Twenty-three full factorial design consisting of experiments for the study of three experimental factors with experimental and predicted values.
Table 3. Percent contribution of each independent variable and their interaction on dependent variable.
Figure 1. Contour plots and three-dimensional (3D) response surface plots showing the effect of number of homogenization cycles and homogenization pressure (A), concentration of stabilizer and number of homogenization cycles (B) and concentration of stabilizer and homogenization pressure on the resultant particle size (C).
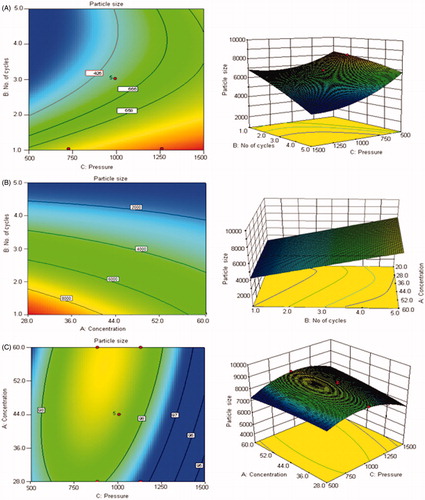
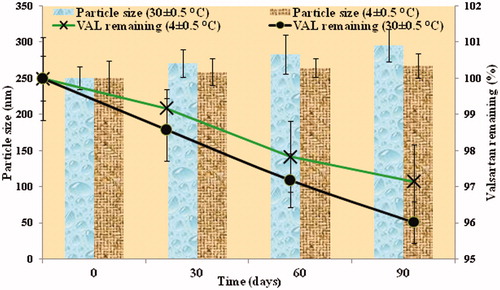
Table 4. Impact of Homogenization cycles and concentration of cryoprotectant on particle size and PDI of formulations obtained by OFAT model.
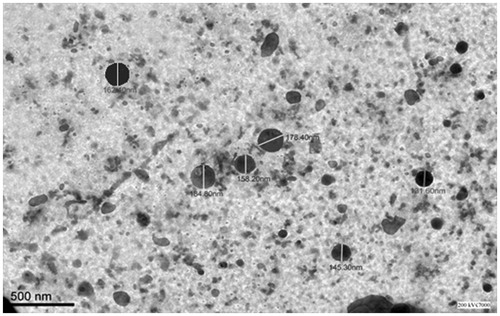
Figure 2. Particle size and PDI of optimized Valsartan nanosuspensions before (A) and after (B) freeze drying.
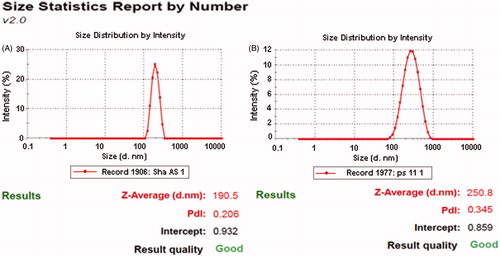
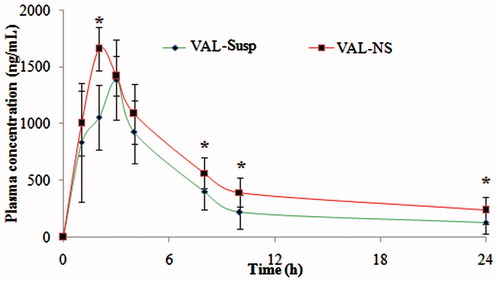
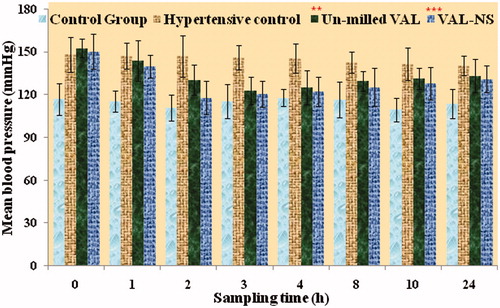
Figure 6. Plasma concentrations after oral administration of micronized VAL (non-homogenized VAL) and VAL nanosuspension (VAL-NS). Here: *p ≥ 0.05 (significant), **p ≥ 0.005 (very significant), ***p ≥ 0.001 (Extremely significant).