Figures & data
Table 1. Quality Target Product Profile (QTPP) for EMLP-loaded MEs.
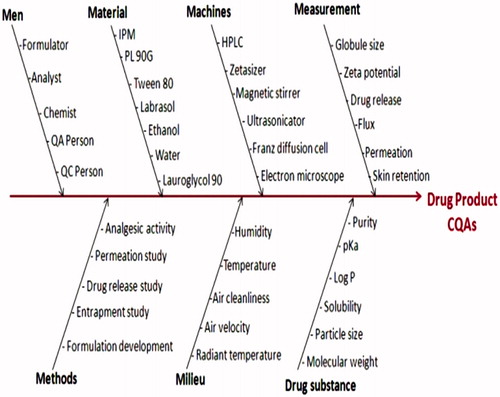
Table 2. Critical Quality Attributes (CQAs) for EMPL-loaded ME and their justifications.
Table 3. Initial risk assessment for ME formulation containing EMLP.
Figure 2. Pseudo-ternary phase diagrams of MEs composed of oil (IPM), Smix (Tween 80: CS: Labrasol®) and water at various Smix ratios (A) 1:1:0.5 (B) 1:1:1 (C) 1:2:1 (D) 2:1:1.
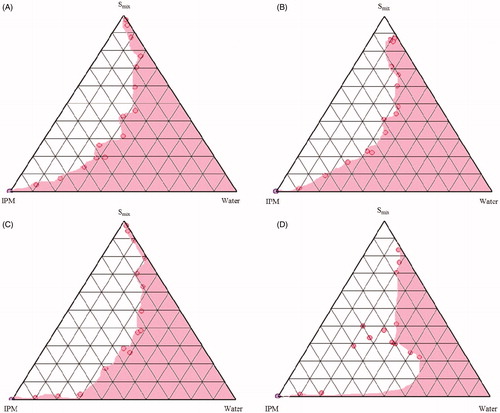
Figure 3. Pseudo-ternary phase diagrams of MEs composed of oil (IPM), Smix (Tween 80: CS: Laurogycol 90®) and water at various Smix ratios (A) 1:1:0.5 (B) 1:1:1 (C) 1:2:1 (D) 2:1:1.
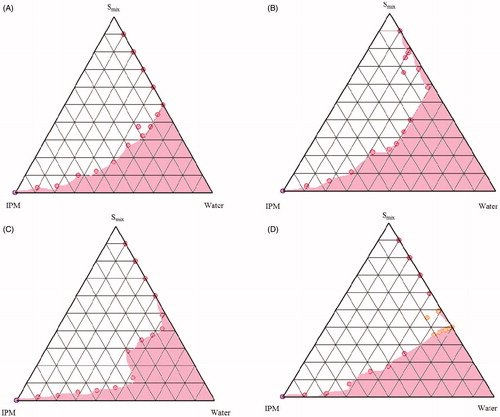
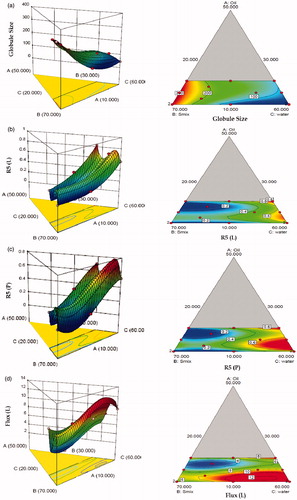
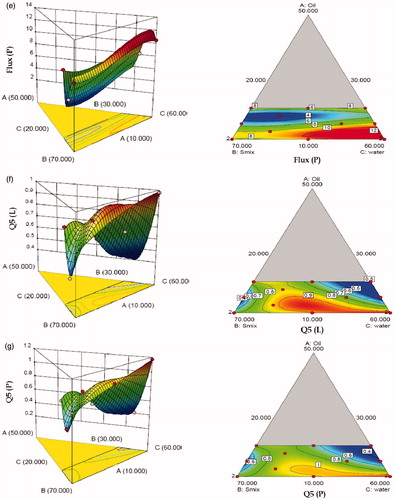
Table 4. Experimental runs of D-optimal mixture design and their responses.
Table 5. Model summary statistics of the measured responses.
Figure 5. Overlay plot depicting the location of optimized microemulsion formulation in the design space.
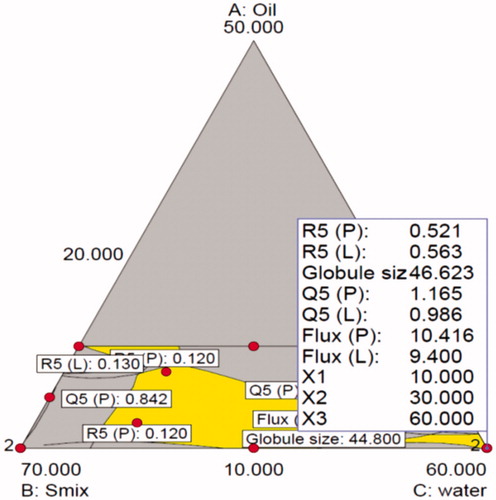
Figure 7. Photograph showing the images of microemulsion formulations (a) MOPT-NMP and (b) MOPT-NMP gel.
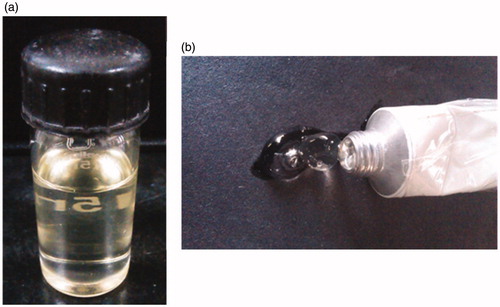
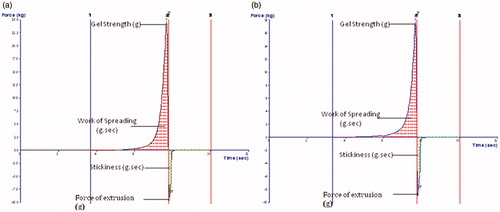
Table 6. Rheological characteristics of the developed ME gel formulations.
Table 7. Chemical stability of Lido and Prilo in the MOPT-NMP gel formulations after 6 months of storage at various conditions. Values are means of three experiments ± SD.
Figure 9. Cumulative permeation amount versus time curves of ME and the reference formulations (a) prilocaine (b) lidocaine (mean + SD); n = 3.
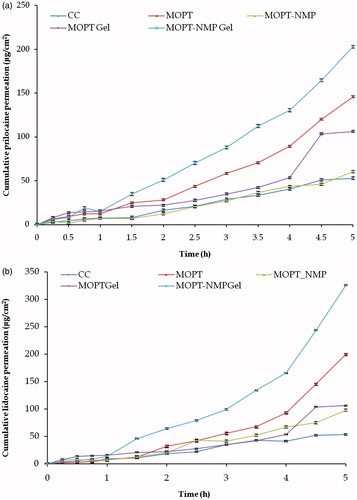
Table 8. Permeation parameters (mean±SD) of lidocaine and prilocaine through rat skin from ME formulations and commercial formulation (n = 3).
Figure 10. Skin absorption of lidocaine and prilocaine from various formulations (mean ± SD); n = 3.
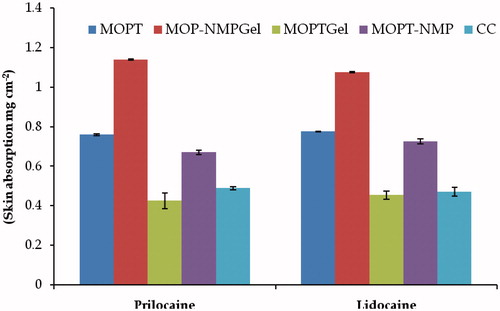
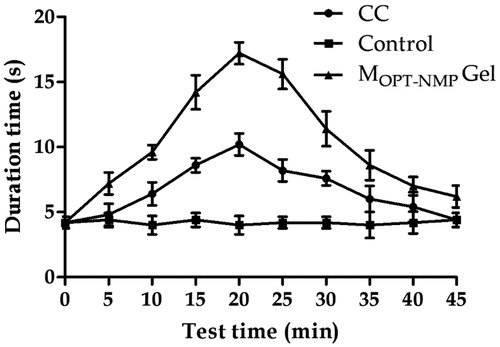
Figure 11. Tail-flick test comparison of control gel, MOPT-NMP gel and commercial cream (CC). The data represent the mean ± SD (n = 5).