Figures & data
Figure 1 (a) Effect of stirring time and speed on particle size and PI. Particle size and PI are affected with the stirring speed and the stirring time. At the stirring speed of 9500 rpm and the stirring time of 2 and 5 min, the average particle size was found to be ∼415 and 350 nm, respectively. At the stirring speed of 16 500 rpm for 2 and 5 min, the size was ∼375 and 525 nm. (b) Effect of stabilizer on particle size and PI. The granulometric distribution of particles is affected by the stabilizer type and concentration, PI of the particles was also affected. (c) Effect of polymer concentration on particle size and PI. Any increase in the concentration of the polymer leads to increase in particle size and PI. (d) Effect of drug concentration on particle size and PI. As the drug concentration is increased, particle size and PI increases thus the particle size and PI are dependent on drug concentration as well.
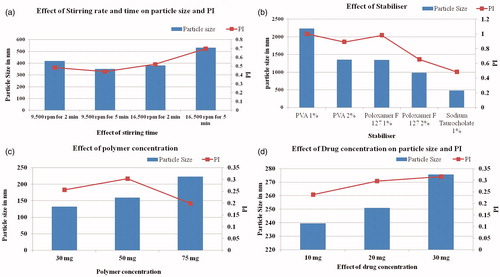
Figure 2. Particle size and PI assessment post-lyophilization of samples. The particle size and PI for the samples before lyophilization were 234 nm and 0.455, respectively. The particle size increased after lyophilization and upon reconstitution in saline and dextrose. It was observed that trehalose is the most suitable cryoprotectant for maintaining the particle size and PI of the particles both pre- and post-lyophilization.
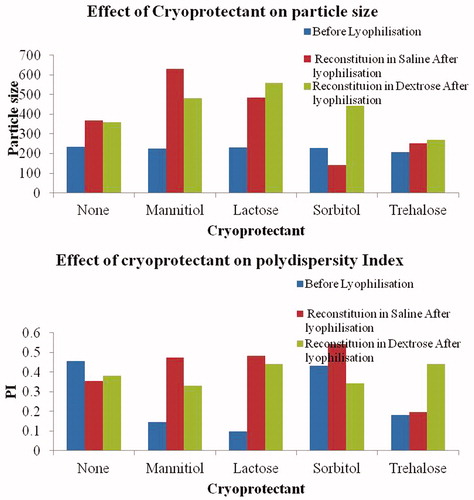
Table 1. Formulations prepared using ethyl acetate as organic solvent and various hydrophilic and hydrophobic stabilizers.
Table 2. Particle size, PI and zeta potential for surface-modified PLA nanoparticles.
Table 3. Zeta potential measurements for designed PLA nanoparticles.
Figure 3. K9 liver cell uptake study performed for surface-modified PLA nanoparticles. Bare nanoparticles due to hydrophobic polymer covering were readily taken showing the highest uptake while least was demonstrated by particles coated with Tween-80 followed by the particles coated with PEG-1000.
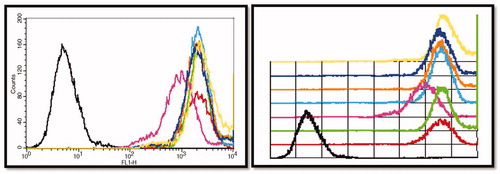
Figure 4. TEM image for TMZ-PLA-NP. Formation of smooth, homogenous and spherical particles was confirmed with TEM analysis as seen in . The particle size (∼200 nm) obtained by the TEM analysis corroborates the findings of dynamic light scattering readings.
Figure 5. DSC thermograms for TMZ, PLA, P-NP and TMZ-PLA-NP. From the DSC thermogram, it was confirmed that the drug was dispersed at molecular level in the formed polymeric matrix. The developed nanoparticles exhibited no interaction between the drug and the excipient.
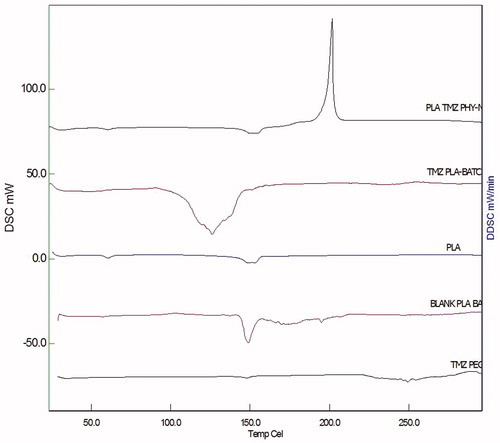
Figure 6. P-XRD pattern for PLA, TMZ, blank and drug-loaded nanoparticulate formulations. P-XRD pattern obtained for the drug demonstrates the highly ordered crystalline nature of the drug. The hollow pattern obtained for the B-NP indicates formation of sub-crystalline or amorphous particles of PLA.
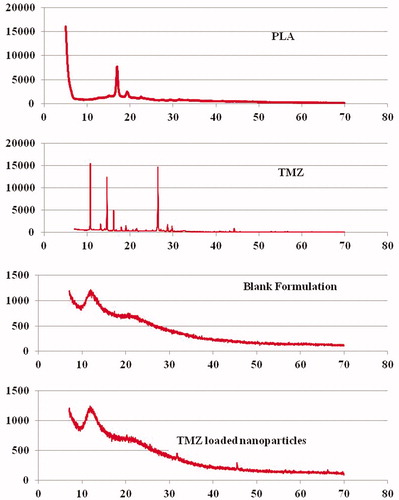
Figure 7. Dissolution profile for TMZ and TMZ-PLA-NP. A biphasic release pattern of TMZ was exhibited from the developed nanoparticles characterized by an initial rapid release of 30% drug during the first 2 h, followed by a slower and continuous release at extremely slow rates for a period of 7 days the release pattern was in respect to the amount of drug encapsulated into the system.
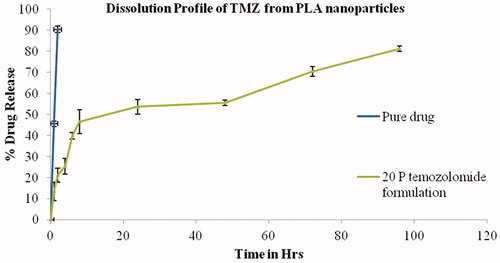
Figure 8. In vitro BBB studies for TMZ and TMZ-PLA formulations. The transport ratios for TMZ-PLA was higher than pure drug through the in vitro co-culture model constructed with U-373 MG cell line and C6 glioma cells at all the tested time-points. The developed nanoparticles transported TMZ through the in vitro BBB model four times better than the pure drug.
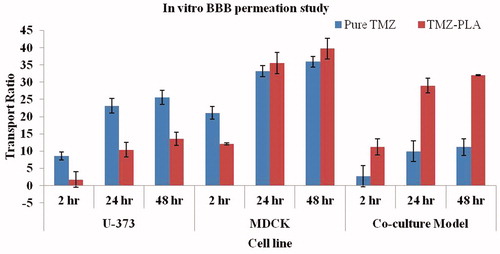
Figure 9. MTT assay for the pure drug and TMZ-loaded PLA nanoparticles assessed on U-87 MG cell line. The developed nanoparticles exhibited time and concentration dependent cytotoxicity.
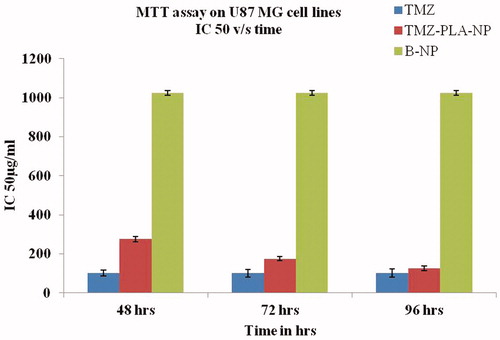
Figure 10. Wound scratch assay performed on U-87 MG cells for pure drug, TMZ-loaded PLA nanoparticle formulation and placebo formulation. Anti-metastatic activity was demonstrated by the developed nanoparticles similar to the pure drug.
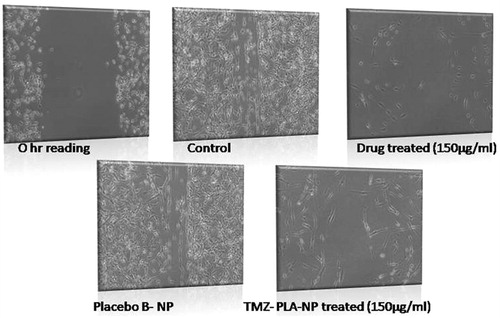
Figure 11. Cellular morphology assay for TMZ and TMZ developed nanoparticles on U-87 MG cell line. Developed nanoparticles exhibited stress on spindle fibers and rounding up of cells similar to the pure drug.
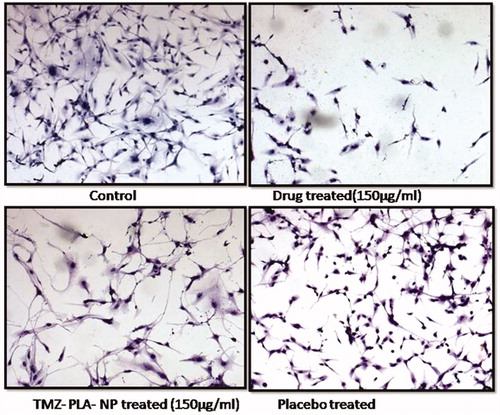
Figure 12. Nanoparticles enhance the cellular uptake by qualitative assessment using confocal microscopy on U-87 MG cells. Developed nanoparticles enhanced the cellular uptake of the particle as indicated by enhanced fluorescence.
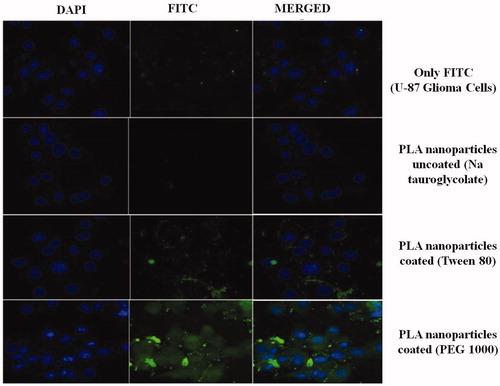
Figure 13. Nanoparticles enhance the cellular uptake in U-87 MG glioma cells by quantitative assessment using flow cytometry. Developed nanoparticles enhanced the cellular uptake of the particle as demonstrated by flow cytometry.
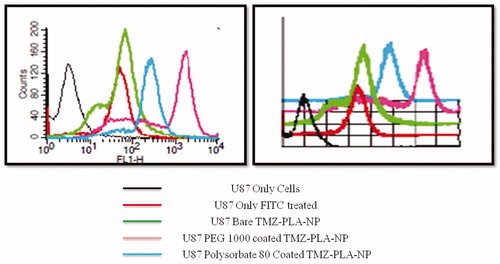
Figure 14. Plasma profile-time concentration plots for TMZ and TMZ-PLA (pharmacokinetic study). Enhancement in half-life (t1/2) AUC was seen for TMZ upon encapsulation in time. The increase in the MRT demonstrated that long-circulating properties were imparted to TMZ and thus prolongation in half-life, better bioavailability and long-circulating properties potentiate the action of TMZ.
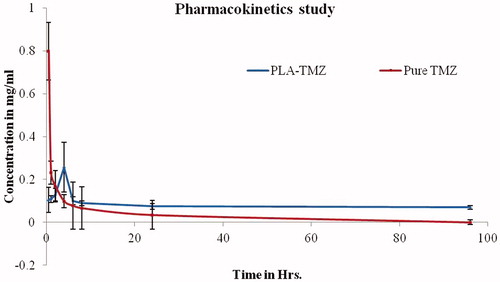
Figure 15. Biodistribution studies for TMZ and TMZ-PLA-NP. The distribution of the drug in brain tissues was four-fold greater with surface-modified nanoparticles as compared to free drug.
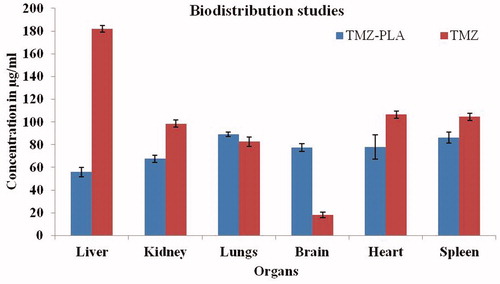
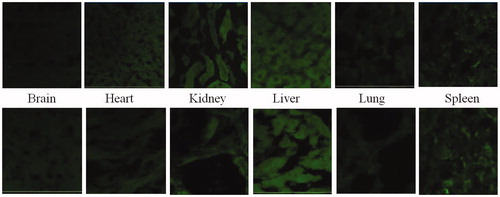
Figure 16. Qualitiative biodistribution studies for pristine dye and dye-loaded PLGA formulations. The fluorescence in the brain section was markedly enhanced for the animals treated with fluorophore-loaded nanoparticles as compared to only dye treated animals. Further, the fluorescence in tissues such as kidneys, liver and lungs was lesser for the animal tissues treated with nanoparticles loaded with fluorophore. Whereas, for organs of animals like spleen and lungs treated with free fluorophore and fluorophore loaded into nanoparticles, fluorescence remained more or less same.