Figures & data
Figure 1. Chemical structures of various vitamin E compounds and isoforms. Illustrating the primary similarities and differences between tocopherols (Tph) and tocotrienols (T3).
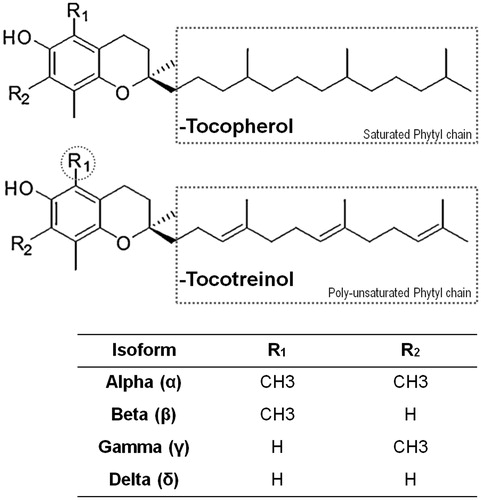
Figure 2. Physico-chemical characterization of different homogenization methods for Tocomin® NE formulation. Change in (A) mean droplet size; (B) Polydispersity Index, PDI; (C) and interfacial electrical charge (measured as ζ-potential), of the various Tocomin® NE homogenization processes. (n = 4–5, mean ± SD values denoted with unlike symbols are statistically different, p ≤ 0.05).
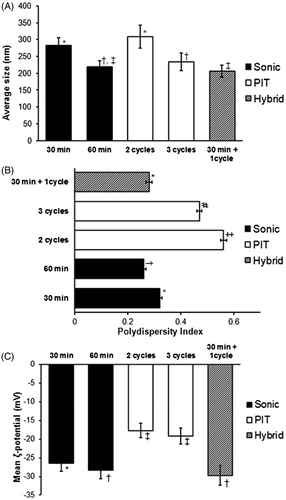
Table 1. Chemical and physical stability of different Tocomin®-NE preparations.
Figure 3. Physical stability of selected Tocomin® NE preparations. Change in mean droplet size, ζ-potential and polydispersity index during (A) cold storage for 60-days, 4–8 °C; (B) mechanical stress test (24 h shaking at 250 rpm, 23 °C); (C) temperature cycling stress test (up to three repeated cycles of freezing–thawing, 24 h each), performed on select Tocomin® NE homogenization processes (n = 4, mean ± SD values denoted with unlike symbols are statistically different, p ≤ 0.05).
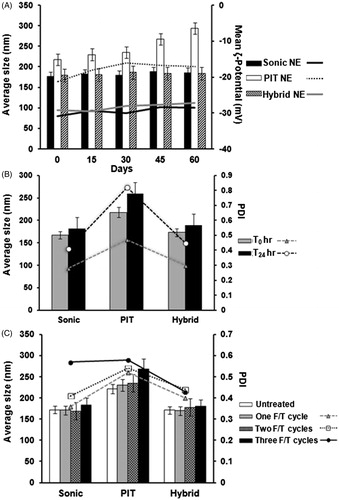
Figure 4. DPPH-antioxidant assay of Tocomin® NE preparations. Homogenized NE formulations of Tocomin® were compared to tocopherol-containing hybrid NE, and Tocomin® mixed with propylene glycol, which serves as non-processes Tocomin® control (n = 4, mean ± SD values denoted with unlike symbols are statistically different, p ≤ 0.05).
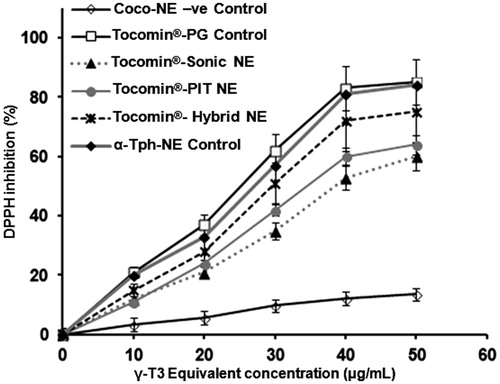
Figure 5. In vitro simulated-skin penetration assay. Franz diffusion cell model data showing (A) time-dependent membrane penetration profile of Tocomin®, over the initial period of 8 h; (B) accumulated amount of Tocomin® after 12 h, calculated based on HPLC-measured γ-T3, for different samples (n = 5, mean ± SD values denoted with unlike symbols are statistically different, p ≤ 0.05).
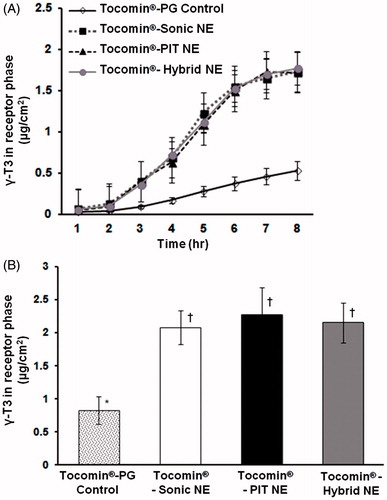
Figure 6. Concentration-dependent anti-proliferative activity of developed Tocomin® NE against skin carcinoma cells. Microplate-based CellTiter Blue® assay of human (A) epidermoid carcinoma, A431; and (B) squamous cell carcinoma, SCC-4, tissue cultures, at γT3-equivalent concentration range of 3–100 μM, and after incubation for 72 h, at 37 °C, 5% CO2 conditions. (n = 5–6, mean ± SD values denoted with unlike symbols are statistically different, p ≤ 0.05–0.01).
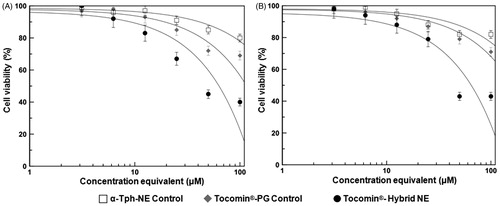
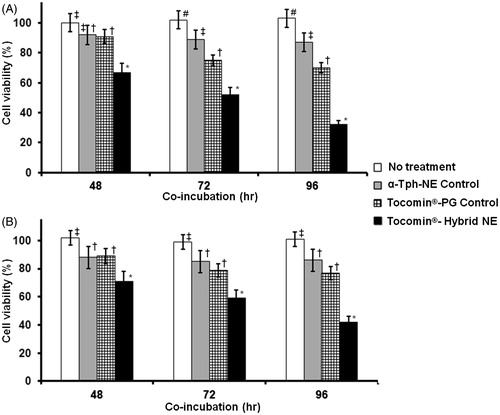
Figure 7. Temporal anti-proliferative activity of developed Tocomin® NE against skin carcinoma cells. Microplate-based CellTiter Blue® assay of human (A) epidermoid carcinoma, A431; and (B) squamous cell carcinoma, SCC-4, tissue cultures, at constant γT3-equivalent concentration dose of 30 μM, measured after different incubation times, 48–96 h, at 37 °C, 5% CO2 conditions (n = 5–6, mean ± SD values denoted with unlike symbols are statistically different, p ≤ 0.05–0.01).