Figures & data
Table 1. Ingredients and concentration using in the formulation of nanoparticles (Mean ± S.D., n = 3).
Figure 2. Atomic force photomicrograph of FDCNs. (a) Single dimension image; (b) 3-D image of FDCNs.
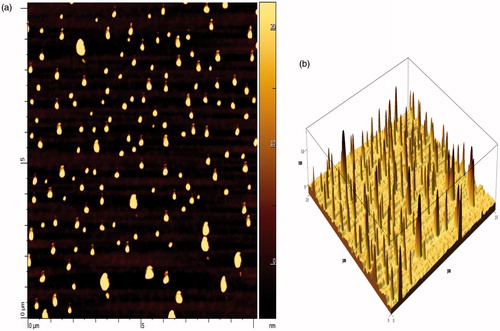
Table 2. Optimization study of different formulations of nanoparticles (Mean ± S.D., n = 3).
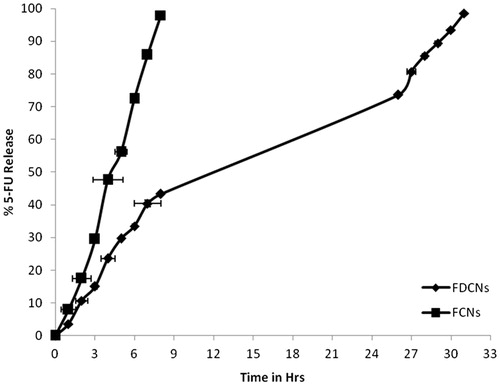
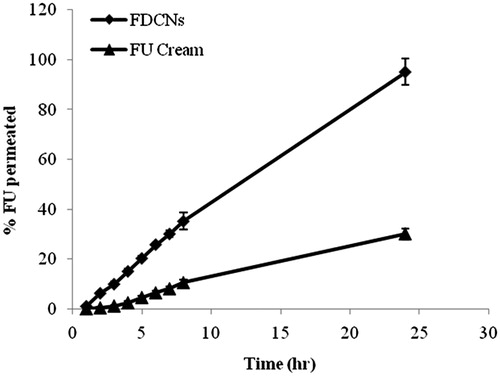
Table 3. In vitro permeation data of 5-FU from the FDCNs, FCNs and FU cream through newly born albino rats (Mean ± S.D., n = 3).
Figure 9. Blood concentration-time profile of 5-FU after applying transdermal cream containing dextran-coated CAP nanoparticles.
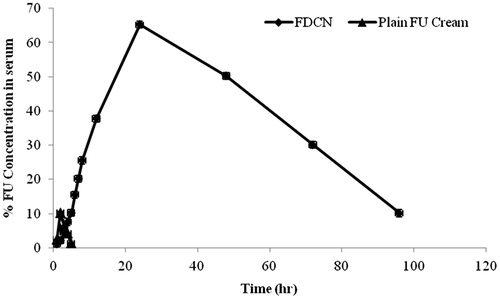
Table 4. Pharmacokinetic parameters of 5-FU from the FDCNs and FU cream through after topical administration albino rats (Mean ± S.D., n = 3).
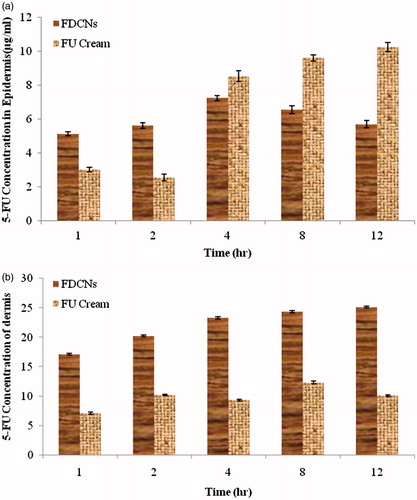
Figure 10. (a) Concentration of 5-FU in epidermis after topical application of FDCNs and FU cream; (b) concentration of 5-FU in dermis after topical application of FDCNs and FU cream.