Figures & data
Figure 3. The antitumor effect of vincristine formulations in SCID mice bearing A431 tumors. The control group (▪) received no treatment and vincristine formulations (vincristine solution (□), DSPC/Chol (○) or SM/Chol (•) liposomal vincristine) were intravenously injected at a dose of 2.0 vincristine mg/kg. Reprinted by permission from Macmillan Publishers Ltd: British Journal of Cancer, 72(4), Webb MS, Harasym TO, Masin D, Bally MB, Mayer LD, Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumor models, 896–904, copyright (Citation1995). We acknowledge Nature Publishing Group's permission (http://www.nature.com/bjc/index.html).
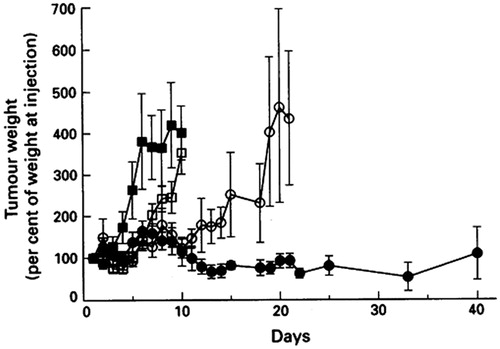
Figure 4. The pharmacokinetics of SM/Chol (○), SM/Chol/PEG-DSPE (•) and SM/Chol/PEG-CER (▪) liposomal vincristine. Concentrations of liposomal lipid (A), vincristine/lipid ratio (B), concentrations of vincristine (C) in plasma at various times after intravenous administration. Reprinted from Copyright Webb et al., (Citation1998), with permission from Elsevier.