Figures & data
Table 1. The experimental plan of the factorial design 23 for the preparation of lacidipine proniosomes for transdermal delivery.
Table 2. The composition of lacidipine proniosomes formulations and their characterization results.
Figure 2. 3D response surface plots showing the effect of independent variables on the mean vesicle size of the prepared lacidipine proniosomes formulations (Y1).
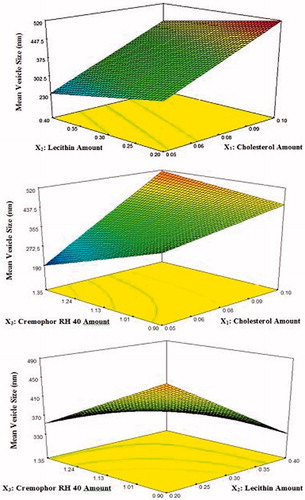
Figure 3. 3D response surface plots showing effect of independent variables on the percentage entrapment efficiency (%EE) of lacidipine in the prepared proniosomes formulations (Y2).
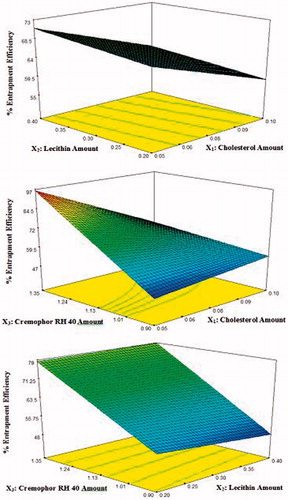
Figure 4. Release pattern of lacidipine from proniosomes formulations and from the plain drug suspension.
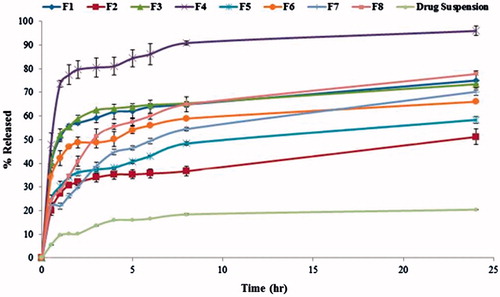
Figure 5. 3D response surface plots showing effect of independent variables on the percentage release efficiency (%RE) of lacidipine from the prepared proniosomes formulations (Y3).
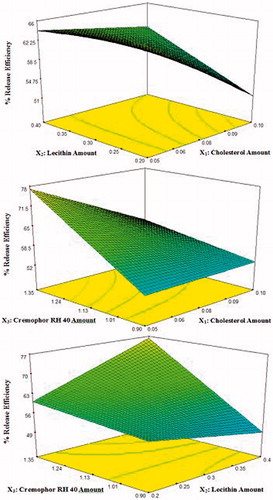
Figure 6. Permeation profile of lacidipine from optimized proniosomal gel formulation and from the control emulgel through excised rabbit skin.
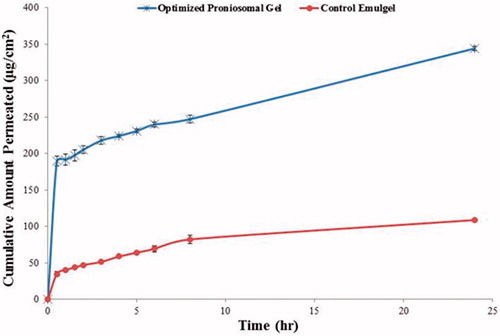
Table 3. Permeation data parameters.
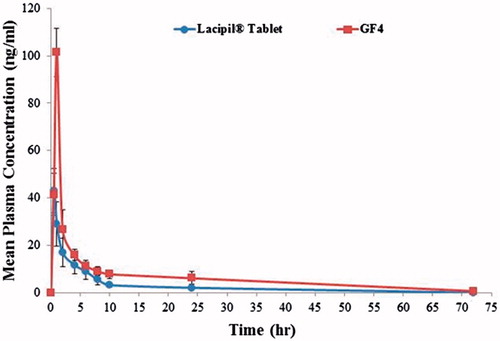
Figure 8: Mean plasma concentration time curve of lacidipine after oral administration of lacipil® tablet and after transdermal application of the proniosomal gel formulation.