Figures & data
Figure 1. Different type of MAs made of silicon, metal, and polymer with microneedles of different shapes. Reprinted with permission from Butler (Citation2015).
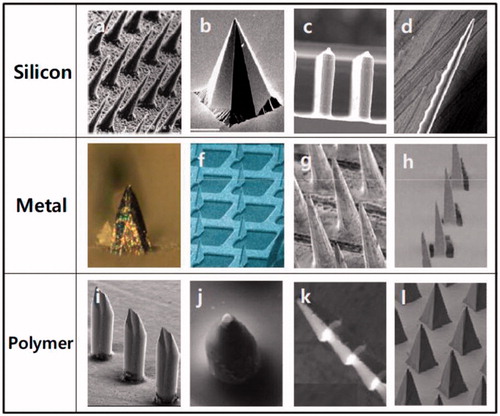
Figure 2. Methods of drug delivery to the skin using different MAs. Microneedles are first applied to the skin (a) and then used for drug delivery (b). Solid microneedle are used as a pretreatment, after which drug can diffuse through residual holes in skin from a topical formulation (solid MA). After insertion of drug-coated microneedles into the skin, the drug coating dissolves off the microneedles in the aqueous environment of the skin (coated MA). Drug-loaded microneedles are made of water-soluble or biodegradable materials encapsulating drug that is released in the skin upon microneedle dissolution (dissolving MN). Hollow microneedles are used to inject liquid formulations into the skin (hollow MA). Reprinted with permission from Butler (Citation2015).
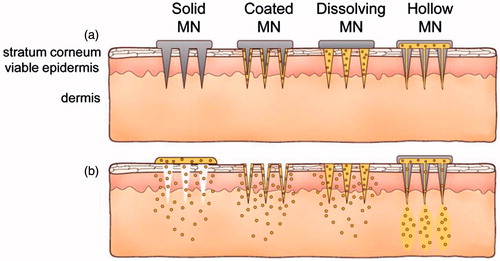
Figure 3. Microneedle patch encapsulating inactivated influenza vaccine. (a) A patch containing a 10 × 10 array of pyramidal microneedle viewed from above. (b) A side view of individual microneedles. Reprinted with permission from Hotez et al. (Citation2016).
Figure 4. A five-needle MA next to a U.S. quarter coin with a diameter of 24 mm. The arrow points at one of the microneedles mounted on the holder. Inset: a single microneedle coated with measles vaccine in a trehalose-based coating formulation. Reprinted with permission from Merkle (Citation2015).
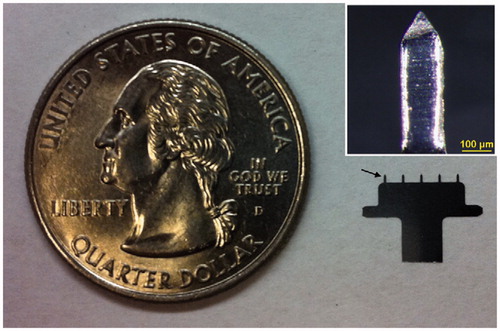
Figure 5. Dissolvable MA for measles vaccination. A microneedle patch is shown next to a 25-gauge hypodermic needle. The patch contains 100 solid microneedles made of water-soluble excipients that encapsulate measles vaccine for delivery to the skin. The inset photo shows a magnified view of the microneedles. To facilitate imaging, the microneedles encapsulated dye (Trypan blue) instead of vaccine. Reprinted with permission from Jacoby et al. (Citation2015).
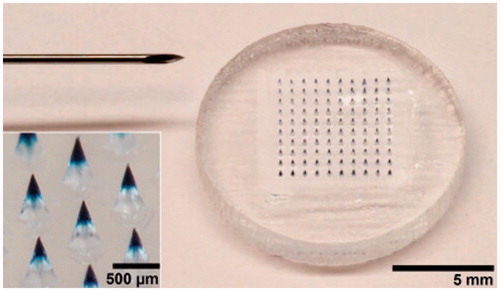
Figure 6. Representative CLSM images of pH-sensitive MA with 24 × 24 microneedles that were coated via a layer-by-layer approach with 10 layers of IPV alternated with TMC: IPV-fluo-488 (a), TMC-rhodamin B (b), and an overlay of these images (c). Reprinted with permission from Neutra & Kozlowski (Citation2006).
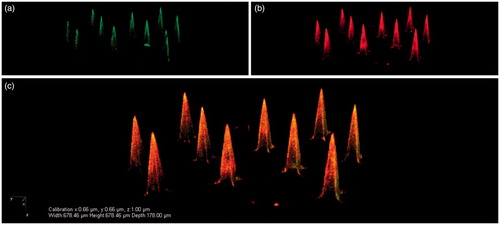
Figure 7. Representative SEM images of platinum thin film-coated silicon MA with 36 pyramidal 180 or 280 μm length MAs. The left image shows a complete MA with 280 μm length microneedles, and the right image is a single-magnified 180-μm microneedle. Reprinted with permission from Resik et al. (Citation2013).
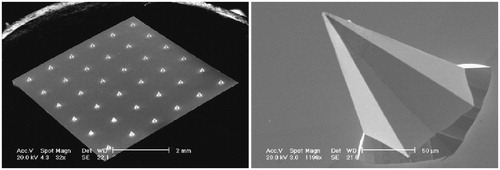
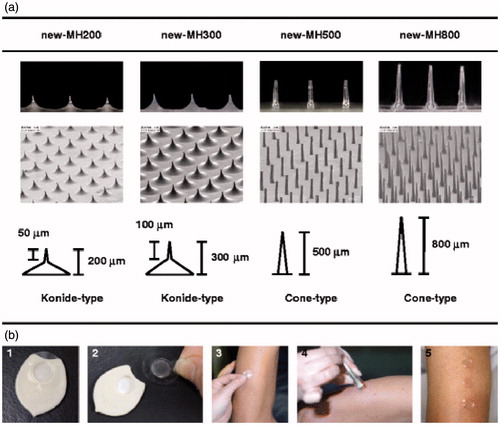
Figure 8. The new-MHs and procedure of new-MH application to the human skin. (a) The new-MHs contain 200 microneedles in an area of 0.8 cm2. New-MH200 and new-MH300 have 200 μm and 300 μm cone-type microneedles of 50 μm and 100 μm lengths, respectively. New-MH500 and new-MH800 contain cone-type microneedles of 500 μm and 800 μm length, respectively.(b) New-MH was fixed to the plastic case as a new-MH formulation (1) was adhered to the center of a 2.3 cm2 adhesive film (2) was put on skin of the lateral upper arm (3) was applied by impact of a handheld spring-type applicator (4) and was removed 6 h after application (5).Reprinted with permission from Hirobe et al. (Citation2013).