Figures & data
Figure 1. SDS-PAGE of merino and kivircik serum paraoxonase. The pooled fractions from ammonium sulfate precipitation and hydrophobic interaction chromatography (sepharose-4B, L-tyrosine, 1-napthylamine) were analyzed by SDS-PAGE (12% and 3%) and revealed by Coomassie Blue staining. Experimental conditions were as described in the method. Lane 3 contained 3 μg of various molecularmass standards: ß-galactosidase (116.0), bovine serum albumin (66.0), ovalbumin (45.0), lactate dehydrogenase (35.0), ∞-lactoglobulin (25.0), lysozyme (19.5).
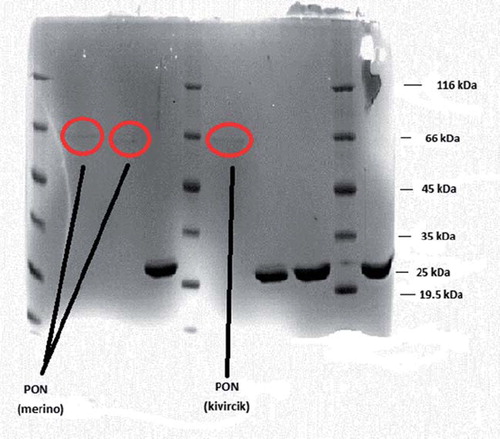
Table I. Summary of the purification of different sheep serum paraoxonase.
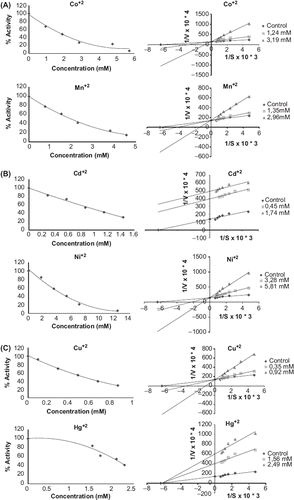
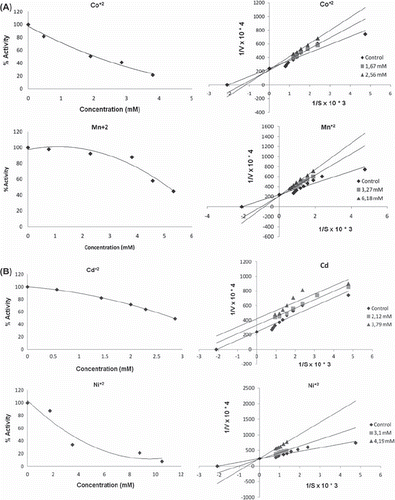
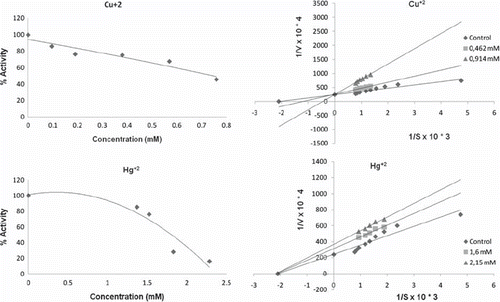
Table II. Type of inhibition and IC50 values (mM) of metals on merino and kivircik paraoxonase enzyme.