Figures & data
Figure 1. (A) Cyclic voltammograms of (a) UCPE, (b) Fe3O4-CPE, (c) BQ-CPE and (d) BQ-Fe3O4-CPE at 10 mVs‐1, (B) curve of the anodic peak current versus the square root of the scan rate for Fe3O4-CPE (inset: cyclic voltammograms at scan rates of 10, 25, 50, 75, 100 and 150 mVs−1) in 0.1 M KCl solution containing 1 mM Fe(CN)63−/4−.
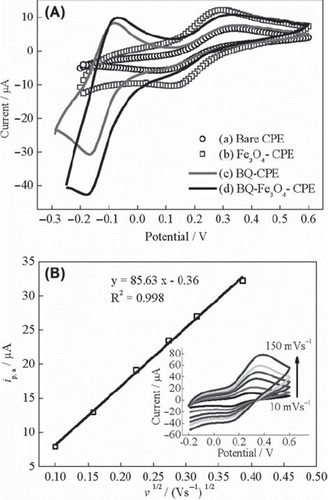
Figure 2. The Nyquist curves of (a) UCPE and (b) Fe3O4-CPE (c) BQ-CPE and (d) BQ-Fe3O4 CPE in 0.1 M KCl solution containing 1 mM Fe(CN)63−/4−.
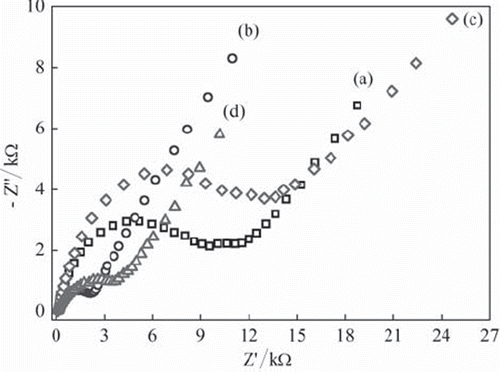
Figure 3. (A) Calibration curves for H2O2: (a) BQ‐Fe3O4‐CPE, (b) Fe3O4‐CPE (c) BQ‐CPE and (d)UCPE (0.05 M pH 7.5 phosphate buffer, + 0.30 V, nitrogen-saturated solution). (B) Calibration curves for glucose: (a) BQ‐Fe3O4‐CPEE, (b) Fe3O4‐CPEE (c) BQ‐CPEE and (d) UCPEE (0.05 M pH 7.5 phosphate buffer, + 0.30 V, oxygen-saturated solution).
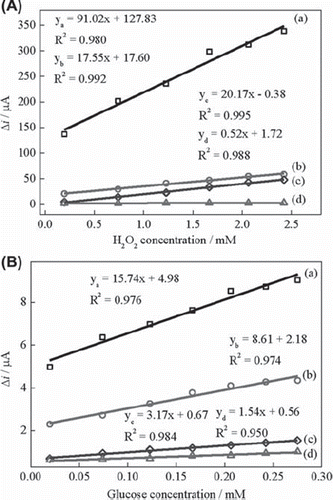
Figure 4. The effect of buffer pH on the response of Fe3O4‐CPEE (0.05 M phosphate buffer, + 0.30 V).
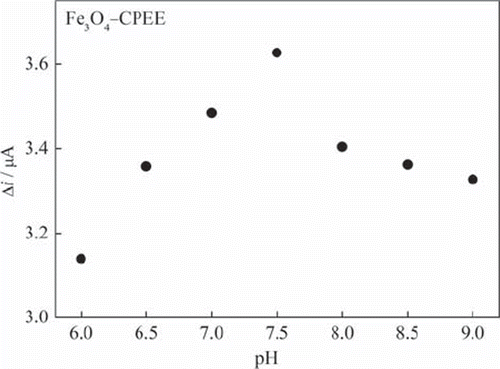
Figure 5. (A) Effect of glucose concentration on the response of Fe3O4‐CPEE (B) Effect of glucose concentration on the response of BQ‐Fe3O4‐CPEE (inset: glucose response of the BQ‐Fe3O4‐CPEE at low concentrations) (0.05 M, pH 7.5 phosphate buffer, + 0.30 V, oxygen-saturated solution).
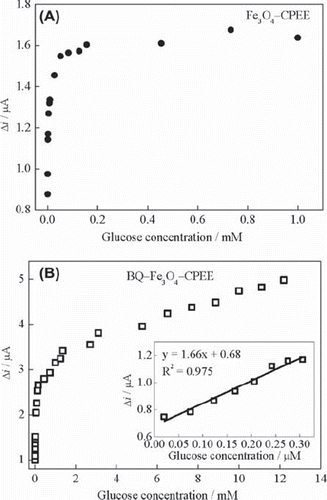
Table 1. Comparison of glucose concentration in serum samples using the modified enzyme electrode and the spectrophotometric method.
