Figures & data
Figure 1 Pooled flow of participants through two studies, one conducted in North America and the other in Europe and Australia.
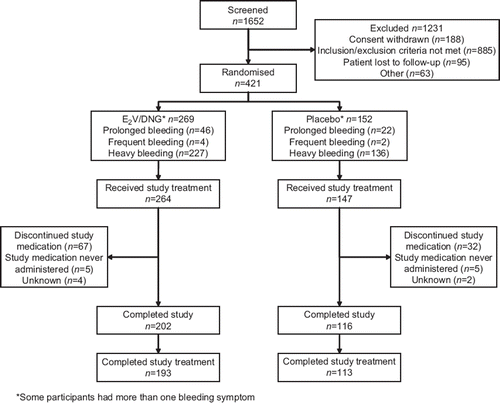
Table 1 Demographic and baseline characteristics of women assigned to oestradiol valerate/dienogest (E2V/DNG) or placebo
Figure 2 Median menstrual blood loss (MBL) by treatment cycle in patients treated with oestradiol valerate/dienogest (E2V/DNG) and placebo in (a) the intent-to-treat (ITT) population and (b) the subgroup of women with excessively heavy menstrual bleeding (defined as MBL greater than 80 ml at baseline; n = 227 for E2V/DNG and n = 136 for placebo). *For comparative purposes, baseline was calculated as (MBL volume during the 90-day run-in phase/90) × 28. **MBL observed during treatment cycle 1 represents the physiological menstrual bleeding (which triggers the start of treatment) plus any intermenstrual bleeding that may have occurred. ***Total blood loss during 28-day treatment cycles.
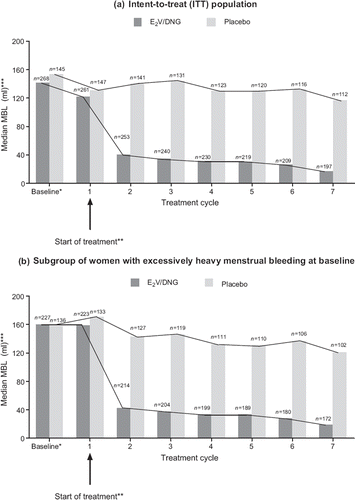
Figure 3 Percentage change in menstrual blood loss (MBL) volume from baseline (90-day run-in phase) to the efficacy phase (90-day efficacy phase) experienced by the proportion of women (intent-to-treat [ITT]).
![Figure 3 Percentage change in menstrual blood loss (MBL) volume from baseline (90-day run-in phase) to the efficacy phase (90-day efficacy phase) experienced by the proportion of women (intent-to-treat [ITT]).](/cms/asset/58d4ad0c-8d92-410b-92c2-d7d1b88a74cc/iejc_a_591456_f0003_b.gif)
Figure 4 Absolute and relative reduction in menstrual blood loss (MBL) according to baseline MBL in the intent-to-treat (ITT) population. Data presented as (a) median reduction in MBL volume from baseline to treatment cycle 7 and (b) median percentage reduction from baseline to treatment cycle 7.
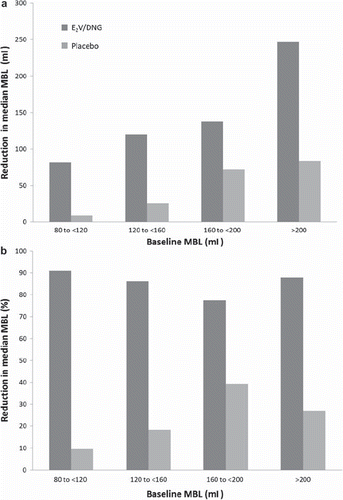
Table 2 Levels of haemoglobin, haematocrit and ferritin at baseline and day 196 of treatment in women who received oestradiol valerate/dienogest (E2V/DNG) or placebo
Table 3 Adverse events that occurred in at least 2% of women who received oestradiol valerate/dienogest (E2V/DNG) or placebo (shown in alphabetical order)