Figures & data
Table 1 Ovarian Activity Grading System.Citation20
Table 2 Demographics and other baseline characteristics.
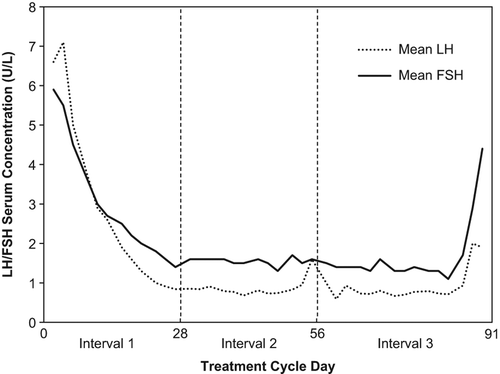
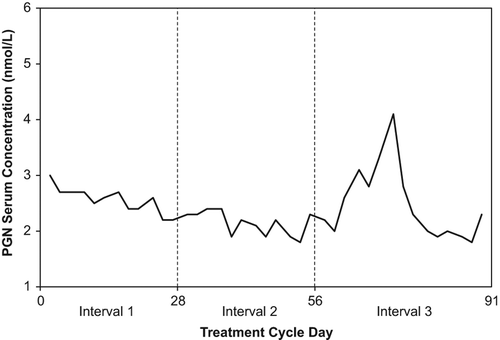
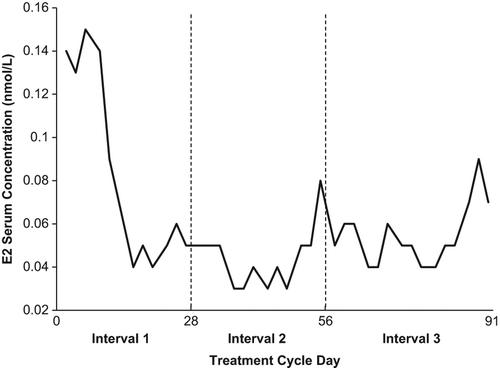
Table 3 Ovarian activity rates (cycle level) for the 91-day treatment cycle converted into three cycle-based intervals of 28 to 35 days: Efficacy cohort.