Figures & data
Table I. Baseline demographic and clinical characteristics in the ITT population, according to the study group.
Table II. Outcomes at 6 months during the randomized phase, according to treatment group.
Figure 1. Changes in outcomes during the trial according to treatment status. Error bars represent standard error (SE). *: p < 0.05 for difference between the two groups. **: p < 0.001 for difference between the two groups. is based on data from individual patients without applying imputation methods.
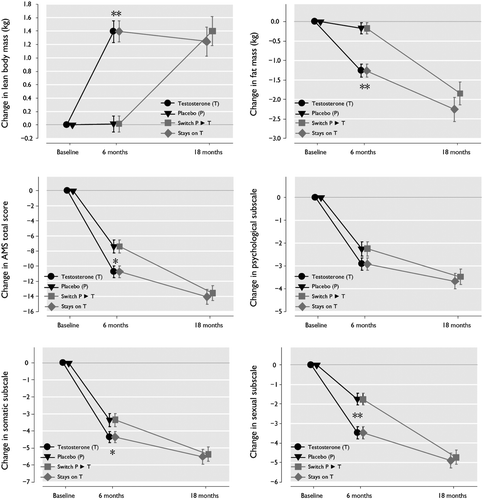
Figure 2. Mean (SD) changes in lean body mass at 6 months with testosterone versus placebo in all patients (a), and in patients grouped by age (b) and by baseline serum total testosterone levels (c).
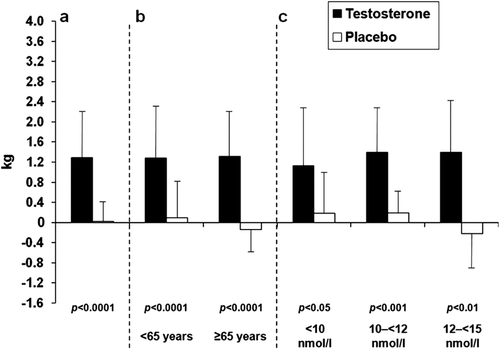
Table III. Effect of Testosterone on the main outcomes at 6 months during the randomized phase, in subgroups.
Table IV. Discontinuations due to increases of serum PSA >1 ng/mL and/or >50% above value at screening.