Figures & data
Figure 1 Study design. All women entered a mono-center, double-blind, placebo-controlled study (Controlled phase) and afterwards women were unblinded to determine the appropriate treatment for the open-label, long-term maintenance study (Open phase). Women having received the test preparation entered directly the maintenance therapy but those from the placebo group first received an initial therapy. The Gynoflor group received vaginal tablets (Medinova AG, Switzerland) containing 0.03 mg estriol and 108 cfu viable Lactobacillus acidophilus. The placebo group received placebo vaginal tablets. E, entry visit; C1, follow-up 5–7 days after start of therapy; C2, follow-up 2–4 days after end of initial therapy; C3, follow-up 2 weeks after start of maintenance therapy; C4, follow-up at end of therapy
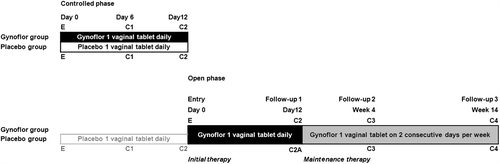
Figure 2 Disposition of subjects, Controlled phase. SAF, Safety population; ITT, intention-to-treat; PPS, per-protocol set
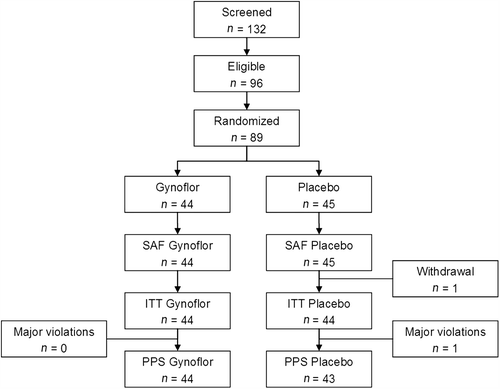
Table 1 Demographic and baseline characteristics
Table 2 Change in Vaginal Maturation Index (VMI), the primary variable, in the Controlled phase
Figure 3 Dynamics of Vaginal Maturation Index (VMI), Controlled phase. E, entry visit; C1, follow-up 5–7 days after start of therapy; C2, follow-up 2–4 days after end of initial therapy
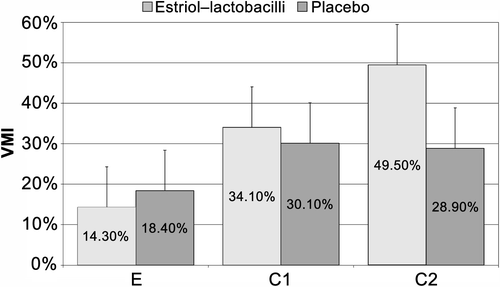
Figure 4 Dynamics of vaginal symptoms, Controlled phase. E, entry visit; C1, examination 5–7 days after start of therapy; C2, examination 2–4 days after end of initial, daily therapy
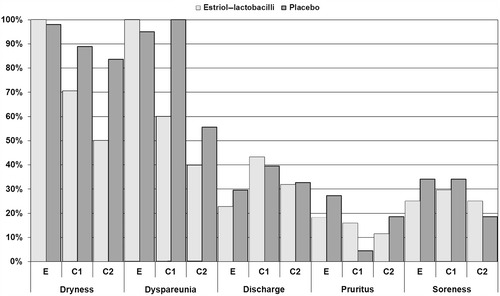
Table 3 Secondary efficacy and safety variables in the Controlled phase. Data are given as mean or %
Figure 5 Dynamics of Vaginal Maturation Index (VMI), Open phase. C2, examination 2–4 days after the end of initial therapy; C3, follow-up visit 2 weeks after start of maintenance therapy; C4, follow-up visit at end of therapy (12 weeks)
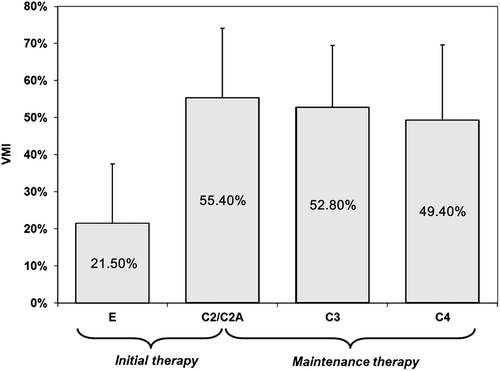
Table 4 Secondary efficacy and safety variables in the Open phase. Data are given as mean or %
