Figures & data
Table 1. Different molar ratio mPEG-SS 5000/NH2.
Table 2. Purification of hen egg white lysozyme.
Figure 1. Polyacrylamide 12% SDS-PAGE electrophoresis of the products of the purification steps of LZ purification. P, molecular mass standard; 1, egg white (20 µg); 2, eluate of IRC-50 (20 µg); 3, permeate IRC-50 (20 µg); 4, eluate of IRP-88 (20 µg); 5, permeate IRR-88 (20 µg). The arrow indicates the LZ band around 14.4 kDa.
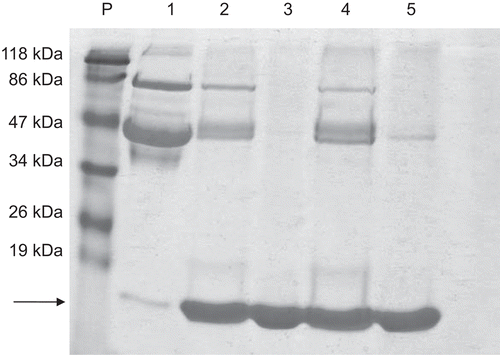
Figure 2. Electrophoresis in polyacrylamide SDS-PAGE 10%. Kinetics of the PEGylation reaction of LZ with mPEG-SS 5000 for 2 h at 30°C. P, standard of molecular mass; 1, 3, 5, 7, 10, 20, 30, 60 and 120 minutes, indicate the time interval in minutes of aliquots collected and submitted to electrophoresis.
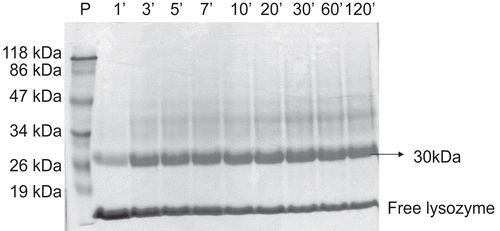
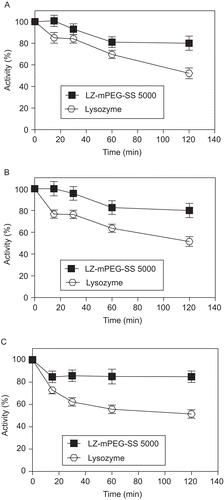
Figure 3. Residual enzyme activity (%) of LZ-mPEG-SS 5000 in different molar ratio (mPEG-SS 5000/NH2) of 0.11, 0.21, 0.43, 0.86 toward substrate. (-•-) M. lysodeikticus; (-▪-) Glycol chitosan. Results are means ± SD, n = 3.
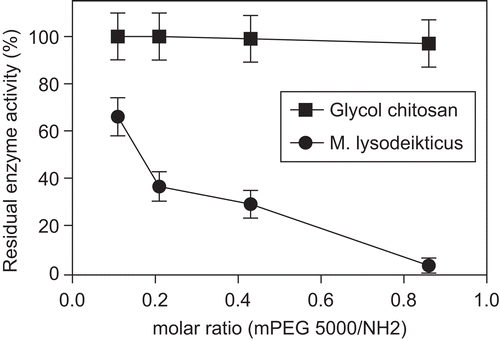
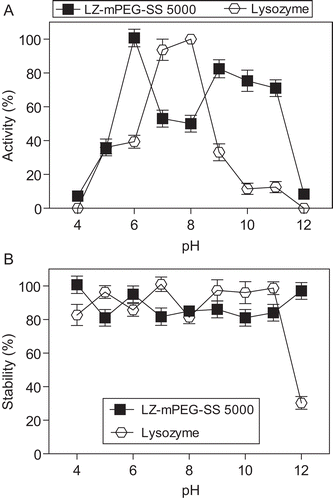
Figure 4. Evaluation of the enzymatic activity (A) and stability (B) of native LZ (-○-) and LZ-mPEG-SS 5000 (-▪-). The activity was determined in different buffers varying the pH from 4–12 (4–5, 50 mM acetate; 6–8, Hepes 50 mM and 9–12 Tris-HCl 50 mM) at 25°C. The stability was determined after incubation by 1 h in the same buffers at 50°C. At the end of this time the enzymatic activity was determined against substrate M. lysodeikticus as described in methods. Results are means ± SD, n = 3.