Figures & data
Figure 1. Influence of C. clathrata hydroalcoholic extract (HE) in nociceptive behavior of mice evaluated in acetic acid-induced abdominal writhing model. Nociception was registered by the number of writhes that the animal presented 20 min following i.p. acetic acid (0.6%) injection. Groups of animals were pre-treated with vehicle (C, control group, n = 6, open column), aspirin (acetylsalicylic acid, ASA, 300 mg/kg, n = 6, cross-hatched column), or HE (100–400 mg/kg, n = 6/group, right-hatched columns), p.o., 60 min before acetic acid. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
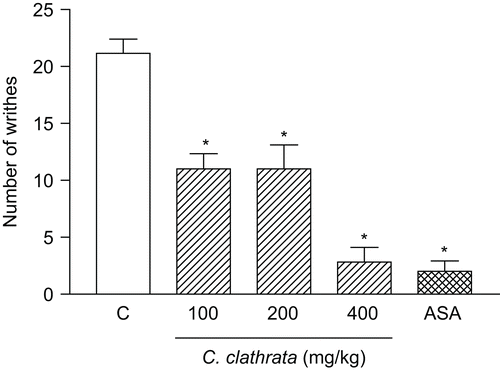
Figure 2. Effect of C. clathrata HE in nociceptive behavior of mice evaluated in formalin-induced nociception model. Groups of mice were pre-treated with vehicle (column C, control group, 10 mL/kg, p.o., 60 min before the administration of formalin, n = 6), aspirin (acetylsalicylic acid, ASA, 300 mg/kg, p.o., 60 min before the administration of formalin, n = 6), morphine (Morph, 10 mg/kg, i.p., 30 min before the administration of formalin, n = 6), or C. clathrata HE (100–400 mg/kg, p.o., 60 min before the administration of formalin, n = 6/group) against the early phase (0–5 min, right-hatched columns) or late phase (20–25 min, cross-hatched columns) of formalin-induced nociception in mice. Each column represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.01 and **p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
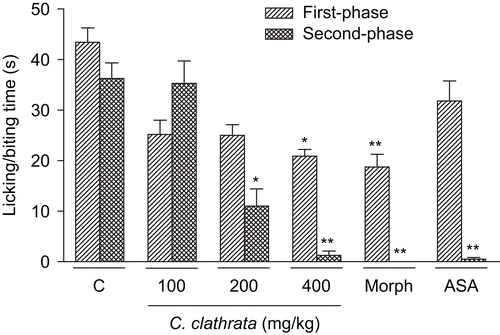
Figure 3. Effect of C. clathrata HE on rat paw edema induced by carrageenan. Groups of rats were pre-treated with vehicle (control group, 10 mL/kg, p.o., n = 6), aspirin (acetylsalicylic acid, ASA, 300 mg/kg, p.o., n = 6), or C. clathrata HE (100–400 mg/kg, p.o., n = 6/group) 60 min before carrageenan-induced paw edema. Measurements were performed at times 0, 1, 2, 3, and 4 h after the subplantar injection of carrageenan (1%, 100 µL). Each value represents the mean ± SEM. Asterisks denote statistical significance, *p < 0.05, **p < 0.01, and ***p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.
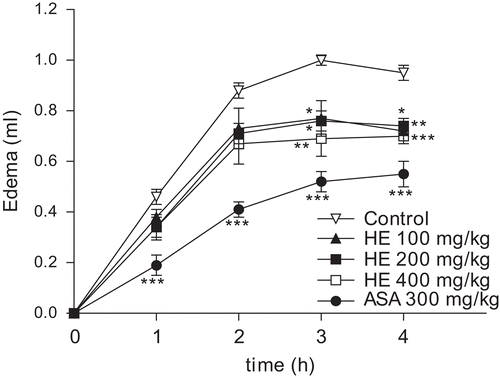
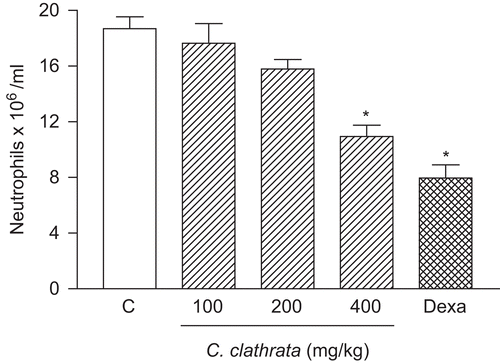
Figure 4. Effect of C. clathrata HE on leukocyte migration into the peritoneal cavity induced by carrageenan in mice. Groups of animals were pre-treated with vehicle (C, control group, 10 mL/kg, p.o., open column), dexamethasone (Dexa, 2 mg/kg, s.c., cross-hatched column), or HE at the doses of 100, 200, and 400 mg/kg (p.o., right-hatched columns) 60 min before carrageenan (1%, 250 μL, i.p.)-induced peritonitis. Cell counts were performed at time 4 h after the injection of carrageenan. Each value represents the mean ± SEM (n = 6/group). Asterisks denote statistical significance, *p < 0.001, in relation to control group. ANOVA followed by Bonferroni’s test.