Figures & data
Table 1. Primer information used in the study.
Figure 1. Impact of the drug on cell viability and proliferation. The cell viability was evaluated with Trypan blue staining. Mock-treated cells and cells treated with maximum nontoxic concentration or higher concentration of phosphonoformate sodium (PFS) were subjected to Trypan blue staining and a representative comparison is provided. (A) The cell proliferation was determined by MTT colorimetric assays. The cell survival rate under different concentrations of drugs is given and 0.85% NaCl (drug dilution solution) was used as control.(B)
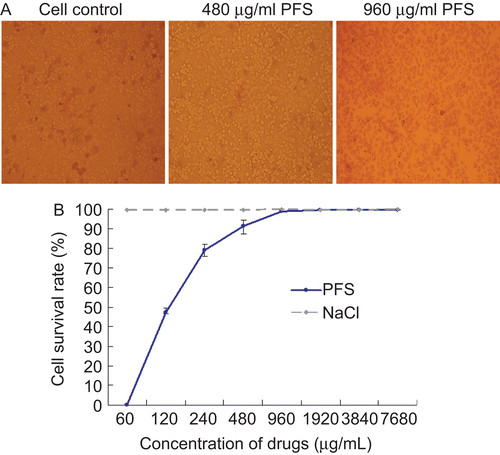
Figure 2. Inhibition ability of phosphonoformate sodium (PFS) to cell infection by pseudorabies herpesvirus (PrV). Increased concentrations of PFS were either incubated with PrV for 1 h prior to cell infection or incubated with PrV-infected cells followed by plaque assays. The plaque inhibition rates calculated from drug-treated virus infection group and drug-treated cells pre-infected with viruses are shown in panels A and B, respectively.
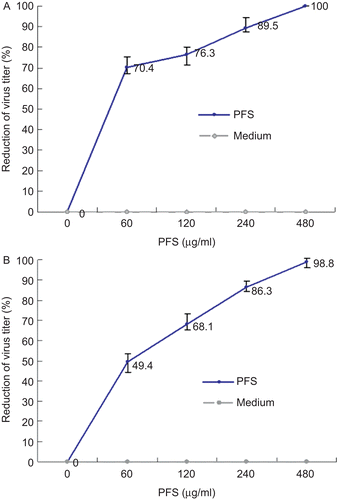
Figure 3. PCR amplification of pseudorabies herpesvirus (PrV) genes. The cells infected by drug-treated viruses or virus-infected cells treated with the drug were subjected to PCR. The effect of phosphonoformate sodium (PFS) on the amount of amplified DNA polymerase, gD, gE, and gG genes are shown. Housekeeping gene GAPDH is served as an internal reference.
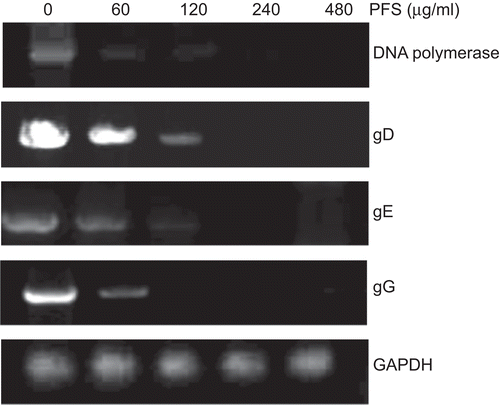
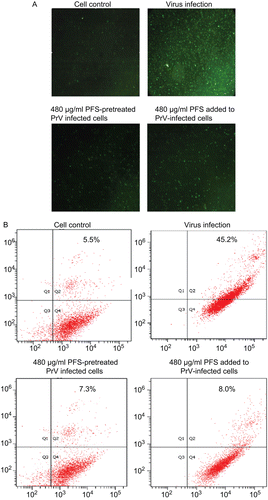
Figure 4. Effect of the drug on cell apoptosis. The cell apoptosis was analyzed after the cells were infected by drug-treated viruses or virus-infected cells were treated with phosphonoformate sodium (PFS). The multiplicity of infection (MOI) of pseudorabies herpesvirus (PrV) used to infect the cells was 2. (A) The green fluorescence signals are designated as the index of early apoptosis in immunofluorescence analysis. (B) To quantify the cell apoptosis, the cells were stained with Annexin V–FITC and PI, either after they were infected by maximum nontoxic concentration of PFS-treated viruses or virus-infected cells were treated with PFS at maximum nontoxic concentration. The cell apoptosis was analyzed by flow cytometry. Data are presented as dual parameter consisting of Annexin V versus PI (Q2 gate). The early apoptosis (Annexin V–FITC staining) and late apoptosis (PI staining) were reflected in Q4 and Q1 gates, respectively. The apoptosis rates of cells are indicated.