Figures & data
Table 1. Descriptions and compositions of the gel samples for rabbit skin irritation test and human skin anti-aging evaluation.
Table 2. DPPH radical scavenging activity of gallic acid and the semi-purified fraction of T. chebula gall extract loaded in non-elastic and elastic niosomes.
Figure 1. Effects of gallic acid at 50 µg/mL and the semi-purified fraction (at 50 µg/mL of gallic acid) of T. chebula gall extract loaded in non-elastic and elastic niosomes on human skin fibroblast viability at 48 h incubation. (BN, blank non-elastic niosomes; BE, blank elastic niosomes; GS, gallic acid solution; GN, non-elastic niosomes loaded with gallic acid; GE, elastic niosomes loaded with gallic acid; SS, the semi-purified fraction; SN, non-elastic niosomes loaded with the semi-purified fraction; SE, elastic niosomes loaded with the semi-purified fraction.)
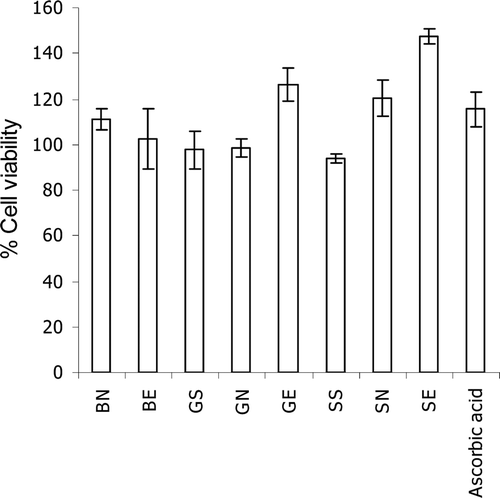
Figure 2. Effects of the gelatinolytic activity on MMP-2 expression of the semi-purified fraction, gallic acid and the standard ascorbic acid at 1–50 µg/mL: (A) zymograms; (B) % relative MMP-2 expression calculated from the following equation: relative MMP-2 expression (%) = (% MMP-2 expression of the sample / % MMP-2 expression of the control) × 100. Each value is expressed as mean ± SD. Kruskall–Wallis test was used to calculate the significant differences. *p < 0.05 and **p < 0.01 compared to each control (the untreated system).
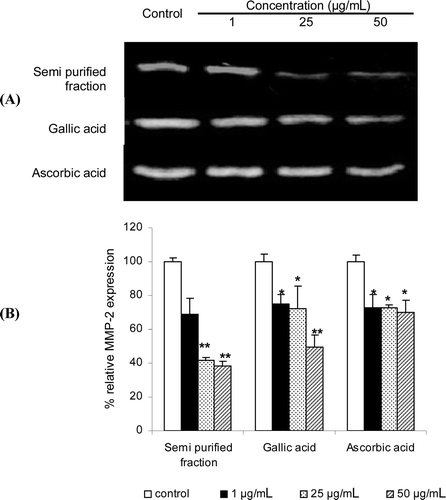
Figure 3. The percentages of gallic acid remaining in various gel formulations at different storage temperatures (27 ± 2, 4 ± 2 and 45 ± 2°C) for 3 months. (GS gel, gel containing the unloaded gallic acid; GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
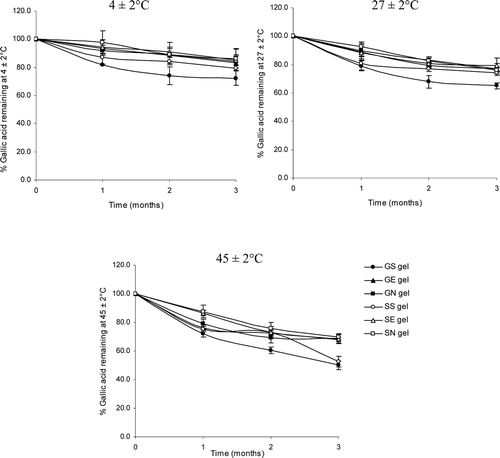
Table 3. Primary irritation index (PII) and category of irritation based on PII of various gel formulations.
Table 4. Percentage changes of skin parameter (%) after 8 weeks of applications of various gel formulations and the negative control (the untreated area).
Figure 4. Changes of the maximum roughness or Rm (A) and the arithmetic average roughness or Ra (B) of various topical gel formulations in 31 human volunteers after topical application for 8 weeks. Student’s paired t-test was used to calculate the significant differences. *p < 0.05 compared to before application (0 wks). (GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
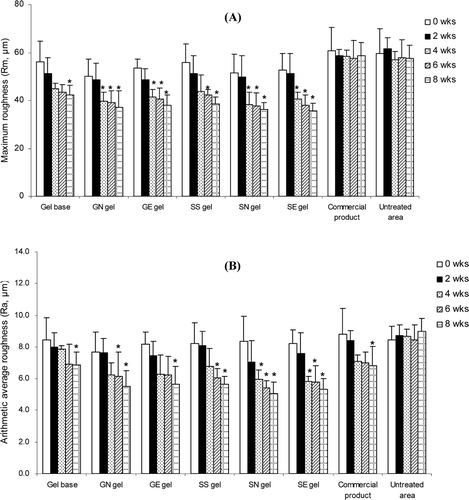
Figure 5. Comparison of the skin roughness before (left) and after application for 8 weeks (right) and % changes of the arithmetic average roughness (Ra) values of various topical formulations. (GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
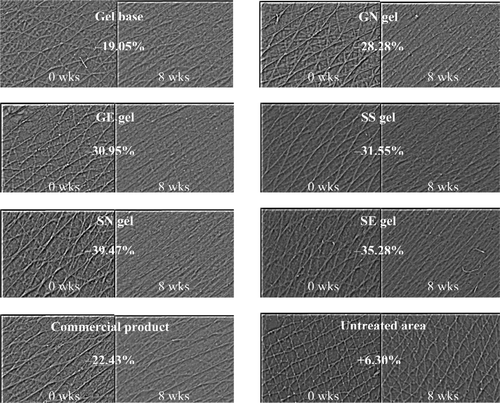
Figure 6. Changes of the skin elastic recovery or Ur/Uf (A) and changes of the skin elastic extension or Uv/Ue (B) of various topical gel formulations in 31 human volunteers after application for 8 weeks. Student’s paired t-test was used to calculate the significant differences. *p < 0.05 compared to before application (0 wks). (GN gel, gel containing non-elastic niosomes loaded with gallic acid; GE gel, gel containing elastic niosomes loaded with gallic acid; SS gel, gel containing the unloaded semi-purified fraction; SN gel, gel containing non-elastic niosomes loaded with the semi-purified fraction; SE gel, gel containing elastic niosomes loaded with the semi-purified fraction.)
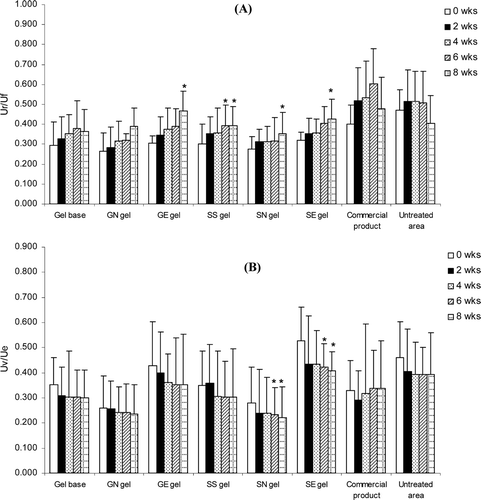
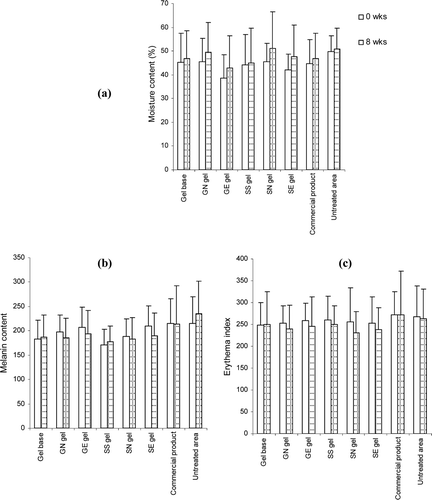
Figure 7. Comparison of moisture (A), melanin content (B) and erythema index (C) on skin surface of various topical formulations at initial and after 8 weeks of application. Student’s paired t-test was used to calculate significant differences. No significant difference was observed between before and after 8 weeks of application (p > 0.05).