Figures & data
Figure 1. Kinetic curve of single CLA isomers against DPPH free radical (a) cis-9, trans-11 CLA (b) trans-10, cis-12 CLA. The final concentrations of CLA were 2.5, 5, 10, 20, 40 and 80 (mg/mL) in the reaction mixtures. DPPH• concentration was 500 µM in all reaction mixtures. Values are mean ± SE (n = 3).
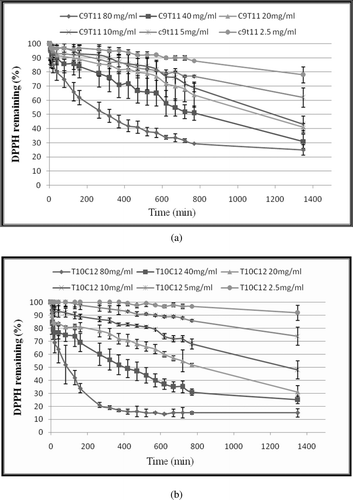
Figure 2. Kinetic curve of mixed CLA isomers against DPPH free radical (a) at ratio 1:6 of trans-10, cis-12/cis-9, trans-11. (b) at ratio 1:13 of trans-10, cis-12/cis-9, trans-11. The final concentrations of CLA were 2.5, 5, 10, 20, 40 and 80 (mg/mL) in the reaction mixtures. DPPH• concentration was 500 µM in all reaction mixtures. Values are mean ± SE (n = 3).
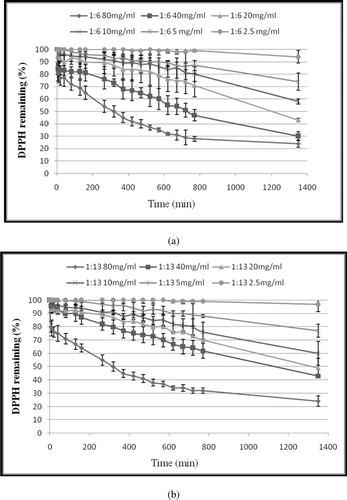
Table 1. Inhibition % of DPPH• radical at steady state among different concentrations of selected CLAs, and median inhibitory concentration (IC50) value for each selected CLA.
Figure 3. Disappearance of DPPH as a function of trans-10, cis-12 and cis-9, trans-11CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) Concentration at steady state of reaction. Vertical bars represent the standard error of each data points (n = 3).
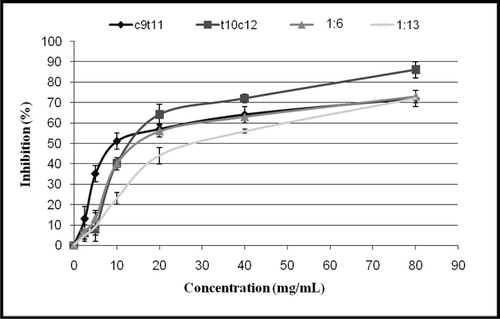
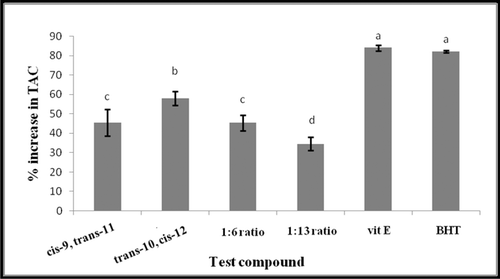
Figure 4. Comparison of TAC of trans-10, cis-12 and cis-9, trans-11 CLA isomers as single or mixed at two ratios, 1:6 and 1:13 (trans-10, cis-12/cis-9, trans-11) with Selected antioxidants vitamin E (Vit E) and butylated hydroxytoluene (BHT). All antioxidants were compared at 50 mM final concentration against 500 µM DPPH radical. Vertical bars represent the standard error of experimental data (n = 3). Columns marked with the same letter are not significantly different (P < 0.05).