Figures & data
Figure 1. H-geniposide and H-catalpol exerted cytotoxicity and suppressed cell proliferation in tumor cells. (A) The chemical structures of iridoid glycosides. (B) After 1 × 104 cells were seeded on 96-well plates, various iridoid glycosides and H-iridoids were treated with 150 µM for 24 h in DU145, MDA-MB-231, and U266 cells. The representative cell viability was accessed using the MTT assay. (C) The cells were treated with various concentrations of H-geniposide (▿) and H-catalpol (▪) for 24 h. The representative cell viability was assessed using the MTT assay; ***p < 0.001 compared to non-treated (NT, •). The representative cell viability was assessed using the MTT assay. (D) After DU145, MDA-MB-231, and U266 cells (5 × 103 cells/well) were seeded onto 96-well plates, they were left non-treated (NT, •) or treated with H-geniposide (▿)and H-catalpol (▪) at 150 µM concentration for the indicated time intervals. The cell proliferation was measured using the MTT assay.
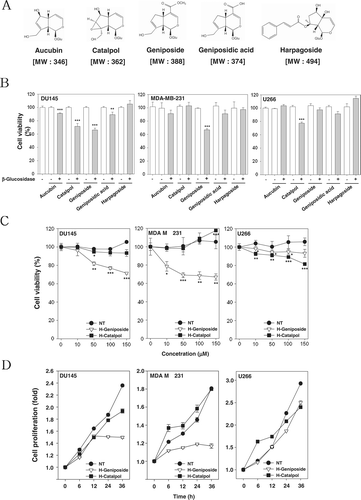
Figure 2. H-geniposide inhibited the upstream signaling molecules of the STAT3 pathway. (A) After DU145, MDA-MB-231, and U266 cells (1 × 106 cells/well) were seeded onto 6-well plates, they were treated with 150 µM of H-geniposide for 24 h. Then, the cells were fixed and observed using a microscope. (B) DU145, MDA-MB-231, and U266 cells (1 × 106 cells/well) were treated with five H-iridoids (150 µM) for 8 h. Whole-cell extracts were prepared and immunoblotted with antibodies for phospho-STAT3 (Tyr705) and STAT3. (C) DU145 cells (1 3 106 cells/well) were treated with H-geniposide (0, 10, 50, 100 mm) for 8 h. Whole-cell extracts were prepared and immunoblotted with antibodies for phospho-STAT3 (Tyr 705) and STAT3. (D) DU145 cells (1 3 106 cells/well) were treated with H-geniposide (0, 10, 50, 100, 150 mm) for 8 h. Whole-cell extracts were prepared and immunoblotted with antibodies for phospho-JAKI (Tyr1022/1023), phospho-JAK2 (Tyr1007/1008), and JAK2 or phospho-Src (Tyr416) and Src.
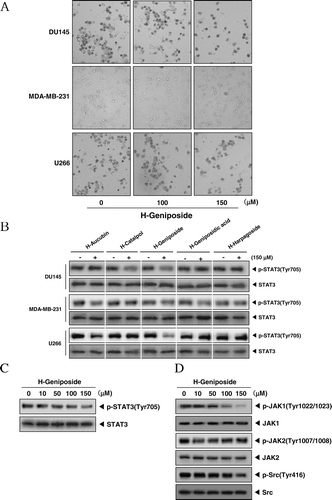
Figure 3. H-geniposide-induced cell cycle arrest and apoptosis. (A) DU145 cells were treated with the indicated concentrations of H-geniposide for 24 h. Thereafter, equal amounts of lysates were analyzed by western blot analysis using antibodies against Cyclin D1, CDK2, and CDK6. The β-actin was used as a loading control (bottom panel). (B) After DU145 (1 × 106 cells/well) were seeded onto 6-well plates, they were left NT or treated with geniposide (150 µM), β-glucosidase, or H-geniposide. After 24 h incubation, the cells were harvested, washed with a cold PBS buffer, and digested with RNase. Cellular DNA staining with propidium iodide and flow cytometric analysis was done to determine the cell cycle distribution as described in the “Materials and methods”. Data are from one representative experiment of the three independent experiments that had similar results among all the results. (C) DU145 cells were treated with H-geniposide for 24 h and the cells were incubated with an FITC-conjugated Annexin V antibody and then analyzed by a flow cytometry as described in “Materials and methods”.
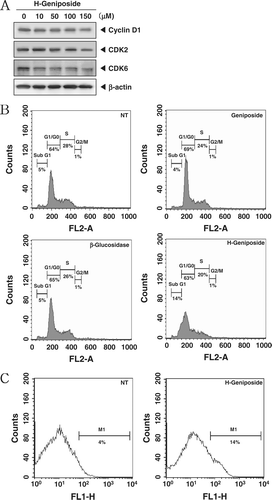
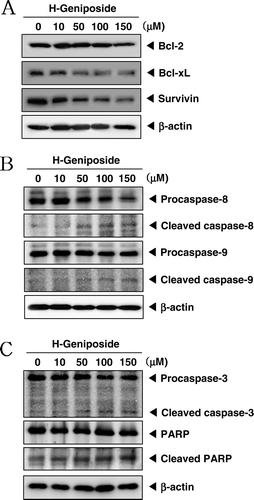
Figure 4. H-geniposide down-regulated the expression of anti-apoptotic gene products and induced apoptosis via caspase-3 activation. (A) DU145 cells were treated with indicated concentrations of H-geniposide for 24 h. Thereafter, equal amounts of lysates were analyzed by western blot analysis using antibodies against Bcl-2, Bcl-xL, and survivin. The β-actin was used as a loading control (bottom panel). (B) Lysates from the cells were subjected to western blot analysis for procaspase-8, cleaved caspase-8, procaspase-9, cleaved caspase-9, and β-actin. (C) Lysates from the cells were subjected to western blot analysis for PARP, caspase-3, and β-actin. A representative blot is shown from the three independent experiments with identical observations.