Figures & data
Figure 1. (a) Schematic representation of an IgG molecule. IgG has a basic four-chain monomeric structure consisting of two identical heavy chains which contains one variable domain (VH) and three constant domains (CH1, CH2, and CH3), and two identical light chains. Between the CH1 and CH2 is the hinge region. An IgG molecule can be divided into two parts functionally: Fragment antigen-binding (Fab) fragment, which is the antigen-binding site, and Fragment crystallizable (Fc) fragment, which is the protein A-binding site (CitationYang et al., 2003). (b) The Staphylococcal protein A shown here is comprised with five homologous IgG-binding domains (E, D, A–C), which have high affnity with the side chains of His435 and Tyr436 of Fc1, and a cell-wall attaching structure (XM) (CitationHober et al., 2007).
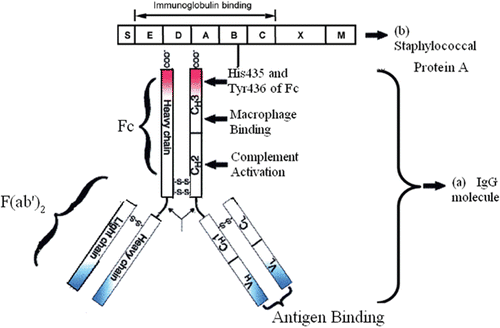
Table 1. Examples of purification of antibodies using synthetic mimic ligands of proteins A and L.
Table 2. Properties of the Ig-binding bacterial proteins A, G and L.
