Figures & data
Table 1. Effects of C. ferruginea on sperm parameters and change in body weight of rats over 90 days.
Table 2. Effects of C. ferruginea on weight of vital organs.
Table 3. Effects of C. ferruginea on organ in vivo antioxidant enzymes and MDA and GSH levels.
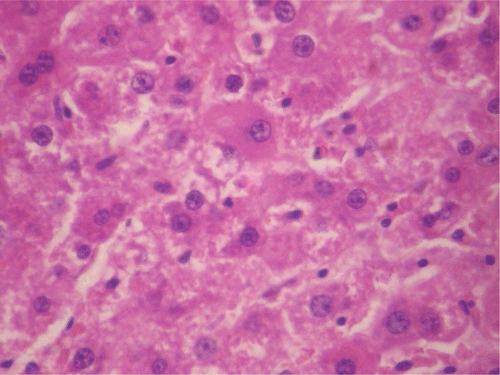
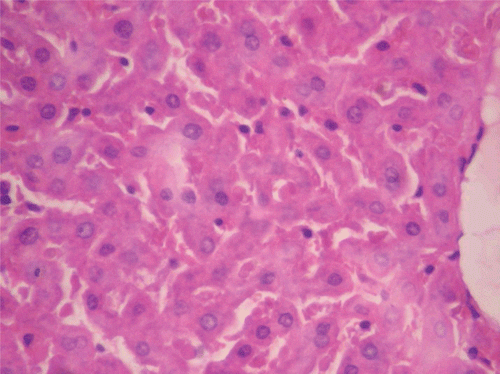
Figure 7. Histopathologic presentation of male rat liver of CF 400 mg/kg group (Intracellular accumulations/Fatty change; 400×).
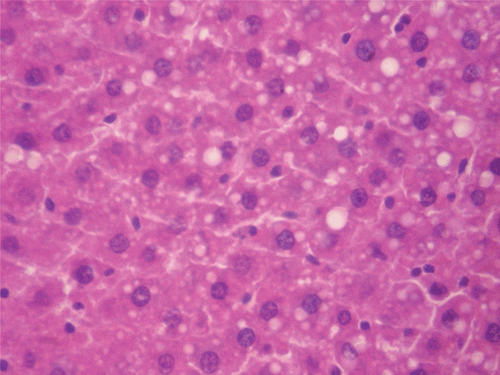
Figure 8. Histopathologic presentation of male rat liver of CF 1000 mg/kg group (Intracellular accumulations/Fatty change; 400×).
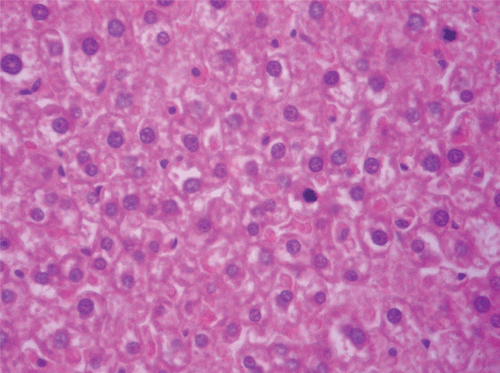
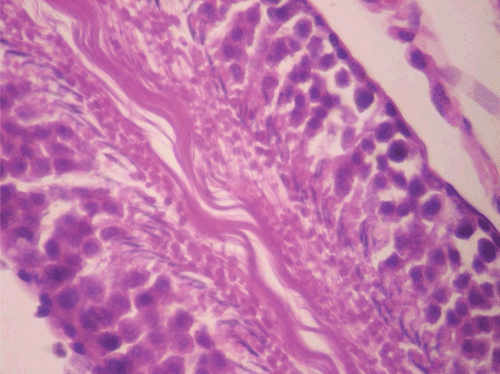
Figure 9. Histopathologic presentation of rat testes of CF 1000 mg/kg group (Inflammation/necrosis; 400×).
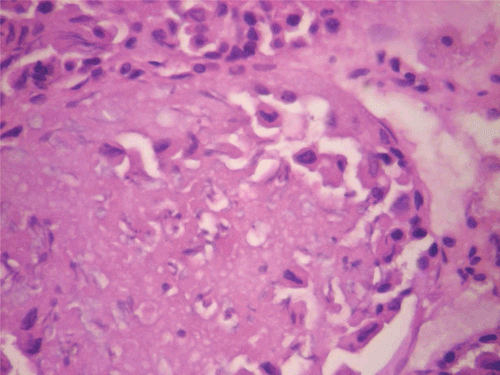
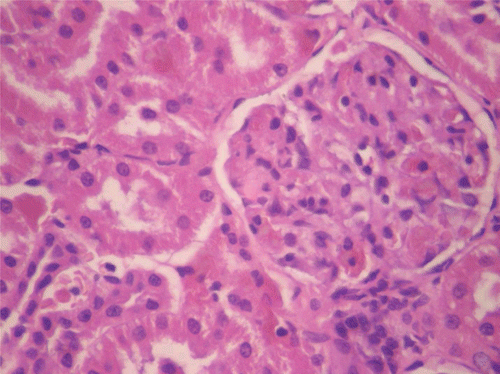
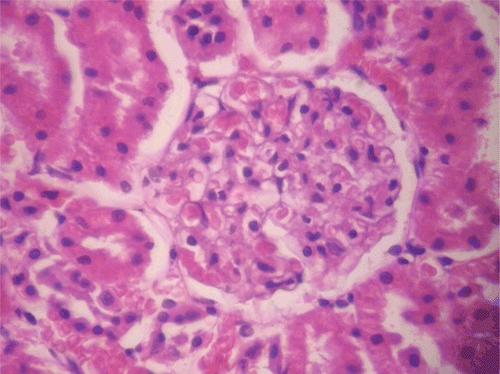
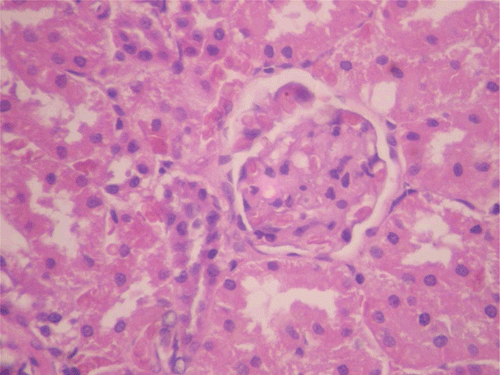
Figure 10. Histopathologic presentation of male rat kidney of CF 80 mg/kg group, reversibility study (Normal; 400×).
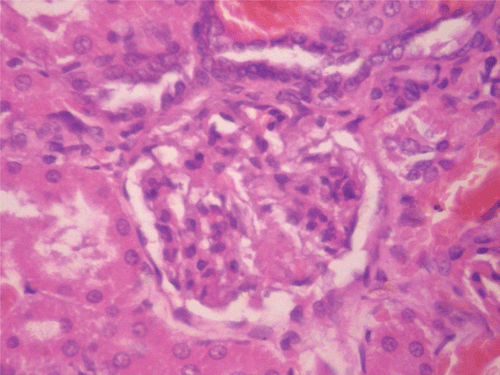
Figure 11. Histopathologic presentation of male rat liver of CF 1000 mg/kg group, reversibility study (Congested; 400×).
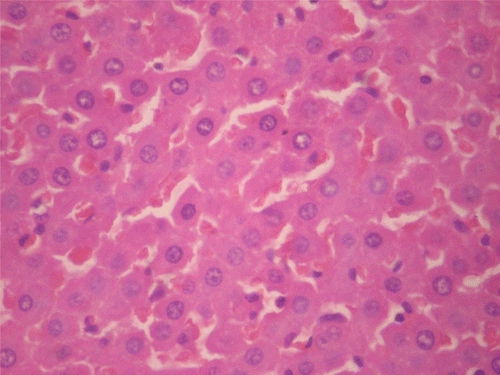
Figure 12. Histopathologic presentation of rat testes of CF 80 mg/kg group, reversibility study (Calcifications; 400×).
Table 4. Effects of C. ferruginea on hematological parameters (main study).
Table 5. Effects of C. ferruginea on hematological parameters (reversibility study).
Table 6. Effects of C. ferruginea on serum biochemical parameters (main study).
Table 7. Effects of C. ferruginea on serum biochemical parameters (reversibility study).