Figures & data
Table 1. Sequences of primers used for RT-PCR.
Figure 1. RT-PCR analysis of TNF-α-induced ICAM-1 expression in HUVEC. (A) Cells were treated with TNF-α (200 U/mL) for 6, 12, 24, 48, 72 h. (B) Cells were treated with various concentrations (20, 100, 200 U/mL) of TNF-α for 12 h. The band intensities were assessed by scanning densitometry. Data were presented as means ± S.D. of three independent experiments. One-way analysis of variance was used to compare the multiple group means followed by Newman–Keuls test (*p < 0.05, **p < 0.01, vs. control group).
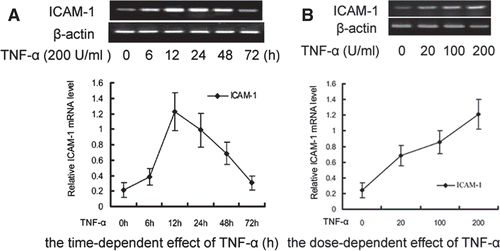
Figure 2. Effects of DTD serum on the expression of ICAM-1 induced by TNF-α through the JNK (A) and p38 (B) pathways. (A) The cells were pretreated with DTD serum (5%, 10%, 20%, 6 h), SP600125 (a JNK inhibitor, 20 µM, 30 min) and then co-treated with TNF-α. (1) Control group, (2) Control serum group, (3) TNF-α group, (4) 5% DTD serum group, (5) 10% DTD serum group, (6) 20% DTD serum group, (7) SP600125 group. (B) The cells were pretreated with DTD serum (5%, 10%, 20%, 6 h), SB203580 (a p38 inhibitor, 10 µM, 30 min) and then co-treated with TNF-α. (1) Control group, (2) Control serum group, (3) TNF-α group, (4) 5% DTD serum group, (5) 10% DTD serum group, (6) 20% DTD serum group, (7) SB203580 group. The band intensities were assessed by scanning densitometry. Data were presented as means ± S.D. of three independent experiments. One-way analysis of variance was used to compare the multiple group means followed by Newman–Keuls test (**p < 0.01, vs. control group; #p < 0.05, ##p < 0.01, vs. TNF-α group).
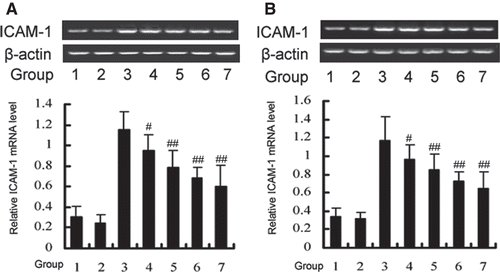
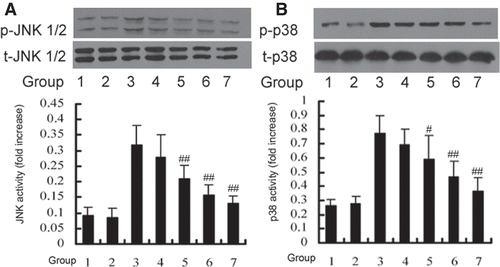
Figure 3. DTD serum inhibited the TNF-α induced activation of JNK (A) and p38 (B). Cells were pretreated with DTD serum for 6 h and then co-treated with 200 U/mL TNF-α for 15 min. Cells were extracted and protein levels were determined by Western blot. DTD serum inhibited phosphorylation of JNK and p38. (A) The cells were pretreated with DTD serum (5%, 10%, 20%, 6 h), SP600125 (20 µM, 30 min) and then co-treated with TNF-α. (1) control group, (2) control serum group, (3) TNF-α group, (4) 5% DTD serum group, (5) 10% DTD serum group, (6) 20% DTD serum group, (7) SP600125 group. (B) The cells were pretreated with DTD serum (5%, 10%, 20%, 6 h), SB203580 (10 µM, 30 min) and then co-treated with TNF-α. (1) control group, (2) control serum group, (3) TNF-α group, (4) 5% DTD serum group, (5) 10% DTD serum group, (6) 20% DTD serum group, (7) SB203580 group. The band intensities were assessed by scanning densitometry. Data are presented as means ± S.D. of three independent experiments. One-way analysis of variance was used to compare the multiple group means followed by Newman–Keuls test (*p < 0.05, **p < 0.01, vs. control group; #p < 0.05, ##p < 0.01, vs. TNF-α group).