Figures & data
Table 1. Table listing both compound and ensemble protein identifiers (CID and Ensemble Protein IDs) for the in silico approach.
Figure 1. EOMI network. An essential oil components-MMP interaction network model was developed by utilizing the STRING 9.0 and STITCH 2.0 database resource search tools, under a score of 0.4 (medium) and a prior correction of 0.0.
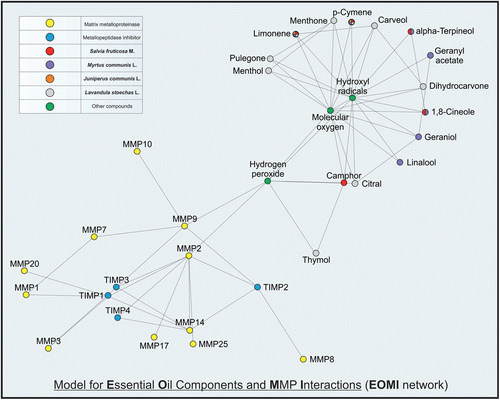
Figure 2. Software analysis (ViaComplex) of the properties within the EOMI network. Figure shows 2D and 3D representations of connectivity (a, b) and clustering coefficient (c, d) of each component in the network model.
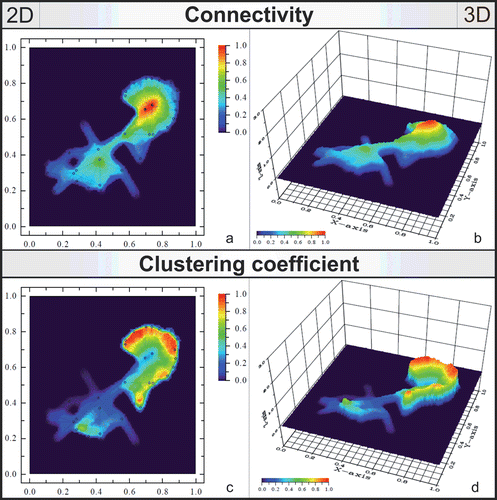
Table 2. Antibacterial effects of essential oils against laboratory (NTCC and ATCC) and clinical (AHN and AHC) strains of six periodontal pathogens.
Figure 3. MIC of the tested essential oils against periodontal bacteria (A.a.: Aggregatibacter actinomycetemcomitans; F.n.: Fusobacterium nucleatum; P.g.: Porphyromonas gingivalis; P.m.: Parvimonas micra; P.i.: Prevotella intermedia; P.n.: Prevotella nigrescens).
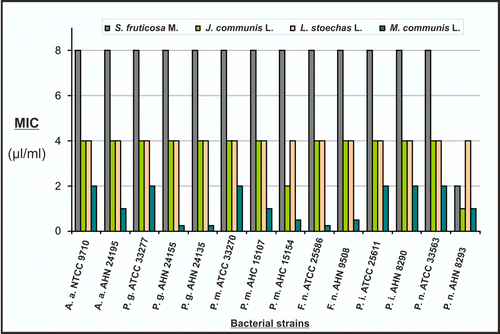
Figure 4. Effect of essential oils on MMP-9 and MMP-2 activities of keratinocytes. HaCaT human skin keratinocytes were treated with essential oils at 1 and 5 µL/mL concentrations in the culture medium, together with 10 ng/mL of PMA, a well-known MMP-9 inducer. Untreated HaCaT and PMA-treated cells represent the negative and positive control, respectively. Gelatinolytic activities seen on 92 and 72 kDa areas represent the MMP-9 (a) and MMP-2 (b) activities, respectively (*Statistical difference with the positive control; p < 0.005).
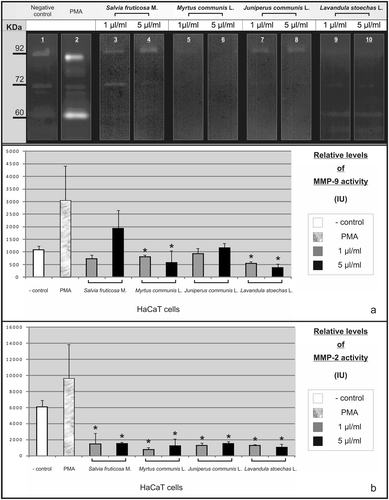
Figure 5. Effect of essential oils on proliferation and viability of HaCaT cells. HaCaT keratinocytes were treated with essential oils at concentrations of 1 and 5 µL/mL. After incubating for 24 h, no significant differences were found in the proliferation (a) and LDH activity (b) of these cells, when compared to control.
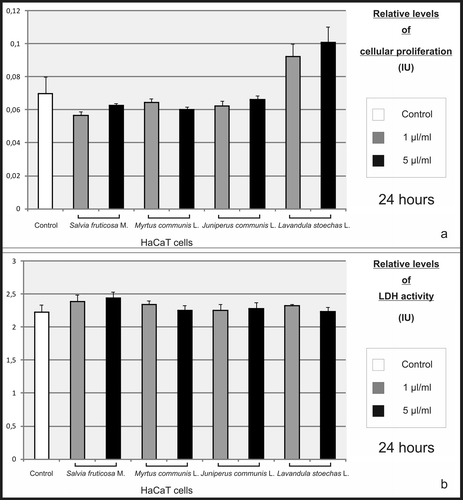
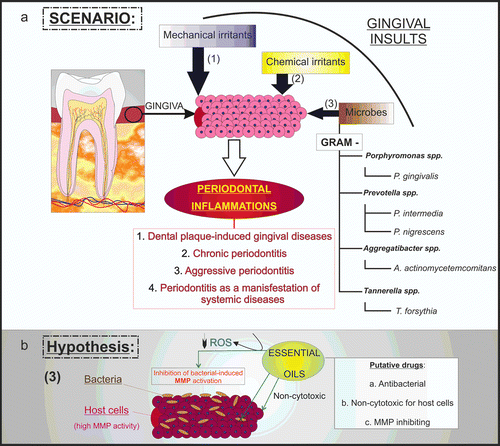
Figure 6. Hypothetical essential oil-induced inhibition in periodontitis. Mechanical and chemical irritants, as well as microbes can trigger periodontal inflammation (a). In such scenarios, specific bacteria exert their virulent effect by inducing host cells to secrete destructive enzymes, such as MMPs. In this work, we hypothesized that essential oil-induced MMP inhibition may occur thanks to the antioxidant properties of these compounds and ROS scavenging (b).