Figures & data
Scheme 1. A schematic representation of two distinct endothelial cell types in adipose tissue. The angiogenesis process which includes endothelial cell growth (proliferation), migration and maturation and leads to vessel formation occurs mainly at the microvasculature level. Differential expression of some growth factors, receptors, enzymes and homeobox genes in various macrovascular and microvascular ECs are also summarized in the table. VEGF, vascular endothelial growth factor; FLK-1, kinase insert domain containing receptor; bFGF, basic fibroblast growth factor; FGFR-1, fibroblast growth factor receptor-1; Ang-1, angiopoietin-1; Tie-1, tyrosine kinase with immunoglobulin-like and EGF-like domain-1; Tie-2, endothelial-specific receptor tyrosine kinase; HLX-1, TLX-1, TLX-2, homeobox genes; Hif-1α, hypoxia-inducible factor-1α (Murakami et al., Citation2001; Murthi et al., Citation2007; Sobrevia et al., Citation2011; Wang et al., Citation2009).
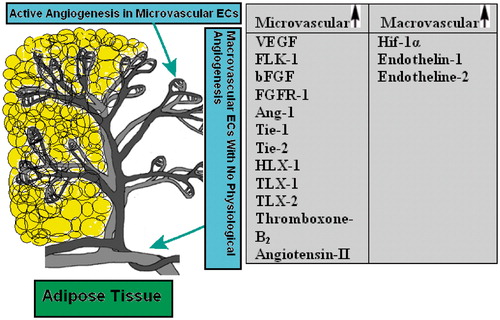
Figure 1. (A) Phase contrast light photomicrograph of confluent monolayer of cultured rat mesenteric endothelial cells (magnification 400×). (B) Immunofluorescent staining of mesenteric endothelial cells for Von-Willebrand factor (magnification 400×). The data are representative of three independent experiments. Further details are given in experimental procedures.
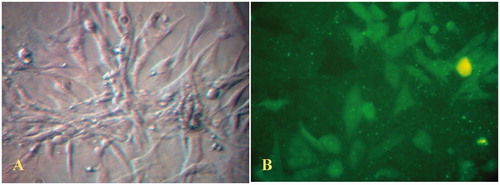
Figure 2. Proliferation kinetics of RMMECs compared with HUVECs. The ECs were synchronized, seeded and allowed to attach overnight in 25 cm2 flasks. Data shown are the mean of ECs number and standard deviations were approximately within 5% of the experimental values of three independent experiments. Further details are given in experimental procedures.
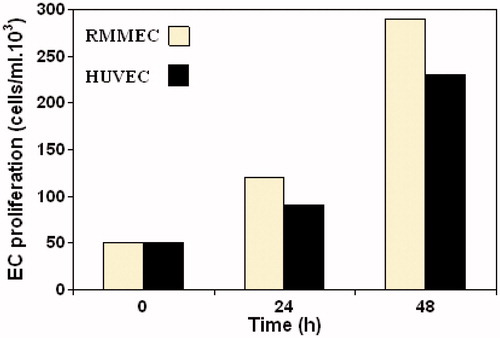
Figure 3. Migration of RMMECs and HUVECs. The representative images of cell migration in the scratch wound-healing model were evaluated under a light microscope at 900× magnification after 24 h of incubation. Red (top) and green (bottom) vertical lines depicted the initially wounded and remained unoccupied regions, respectively. Data shown are representative of the results of three independent experiments. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
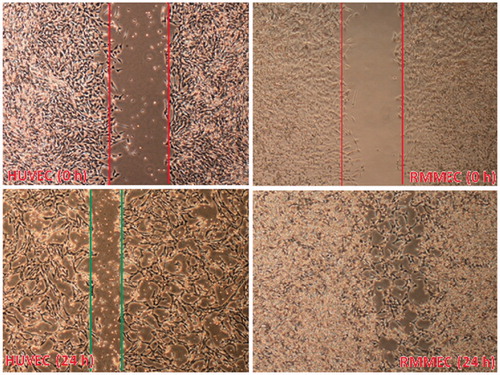
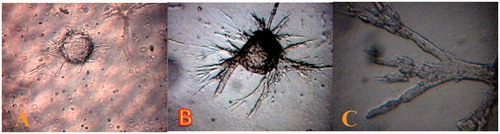
Figure 4. Rat mesenteric endothelial cell for in vitro angiogenesis. (A) Spontaneous formation of capillary-like structures by mesenteric ECs on the microcarriers. Microcarriers seeded with rat mesenteric endothelial cell were embedded in collagen matrix. The endothelial cell attached to particles has been migrated through the collagen matrix (B) total explants area and (C) more details of outgrowth area, in which endothelial cells are seen to form tube-like structures (magnification 400×). The data are representative of 2–3 independent experiments. Further details are given in experimental procedures.