Figures & data
Figure 1. Accelerated stability analysis of α-ketoglutarate (α-KG) formulation was carried out for a period of 6 months. At the beginning of the experiment (“0” month), absorbance of α-KG was recorded at 240–400 nm. The UV spectrum of α-KG was recorded at 317 nm, while cyanide did not show any absorbance at 240–400 nm. Thereafter, cyanide and α-KG were mixed in 1:1 ratio and the disappearance of α-KG peak was observed, which confirmed the interaction of α-KG and cyanide to produce cyanohydrin complex.
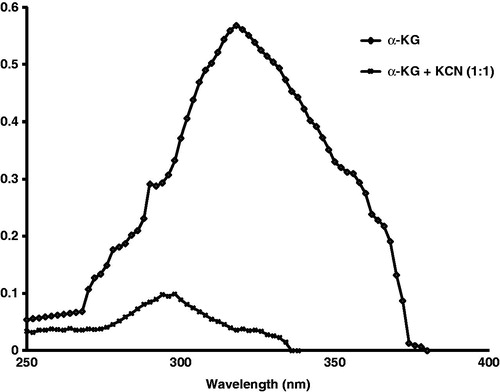
Table 1. Various physical, chemical and microbiological assays carried out during accelerated stability analysis of α-KG formulation.
Table 2. Protective efficacy of different doses of α-KG formulation against acute cyanide intoxication in mice.
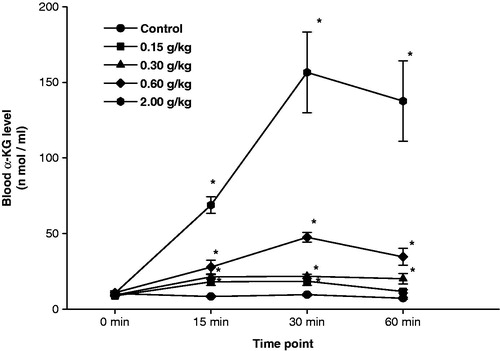
Figure 2. Accelerated stability analysis of α-ketoglutarate (α-KG) formulation was carried out for a period of 6 months. At the beginning of the experiment (“0” month), female rats were administered α-KG (oral) formulation at 0.15 g/kg (low dose), 0.30 g/kg (medium dose), 0.60 g/kg (high dose) and 2.0 g/kg (protective dose). Thereafter, blood α-KG levels were measured at various time intervals. Values are mean ± SE of six animals. *Significantly different from corresponding control at p < 0.05.