Figures & data
Figure 1. Representative traces of ginsenosides on the contractile amplitude of isolated jejunal segment (IJS). NC, normal control, the contractility recorded in normal contractile state. Contractile amplitude of IJS in the normal contractile state is set to a relative value of 100% (normal control, NC). Data are expressed as the mean ± SEM. (% NC, n = 6); **p < 0.01 compared with NC.
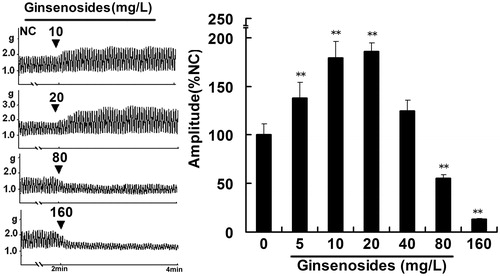
Figure 2. Ginsenoside-induced bidirectional regulation (BR) on the contractility of isolated jejunal segment (IJS). Representative traces and statistical analysis of total traces from six independent experiments of ginsenosides-induced BR on the contractility of IJS in (A) six low contractile states (LCS) and in (B) six high contractile states (HCS). Contractile amplitude of IJS in the normal contractile state is set to a relative value of 100% (normal control, NC). Low and high contractile states of IJS are the relative values compared with NC. Data are expressed as the mean ± SEM. (% NC, n = 6); ##p < 0.01 compared with nc; **p < 0.01 compared with contractile amplitude of IJS in LCS or HCS before given ginsenosides, respectively. CP: Constipation-prominent rats; DP: Diarrhea-prominent rats; SNP: Sodium nitroprusside.
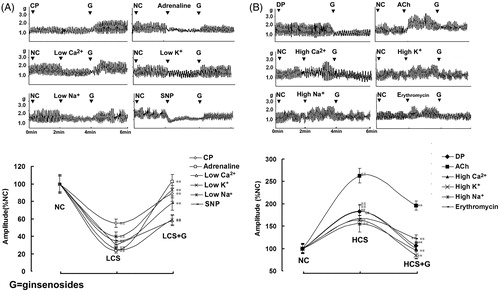
Figure 3. Effects of ginsenosides on the contractility of isolated jejunal segement (IJS) pretreated with tetrodotoxin (TTX). Contractile amplitude of IJS in the normal contractile state is set to a relative value of 100% (normal control, NC). Other data are the relative values compared with NC. Data are expressed as the mean ± SEM (% NC, n = 6); *p < 0.05, **p < 0.01 compared with contractile amplitude of IJS after treatment with TTX (0.1 µmol/L). RLCS: Representative low contractile state; RHCS: Representative high contractile state.
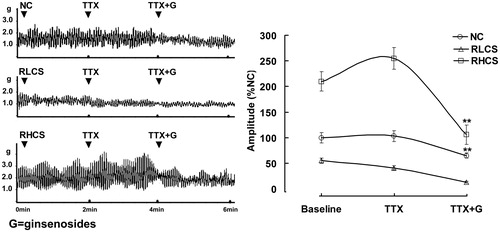
Figure 4. Effects of ginsenosides on the contractility of isolated jejunal segement (IJS) pretreated with receptor antagonist. Effects of ginsenosides on the contractility of IJS pretreated with (A) Ca2+ free Krebs’ buffer or verapamil (1 µmol/L) (B) 10 μmol/L atropine and 10 μmol/L diphenhydramine (in the representative low contractile state, RLCS); 10 μmol/L phentolamine, 5 μmol/L propranolol and 10 μmol/L l-NG-nitroargenine (l-NNA) (in the representative high contractile state, RHCS).
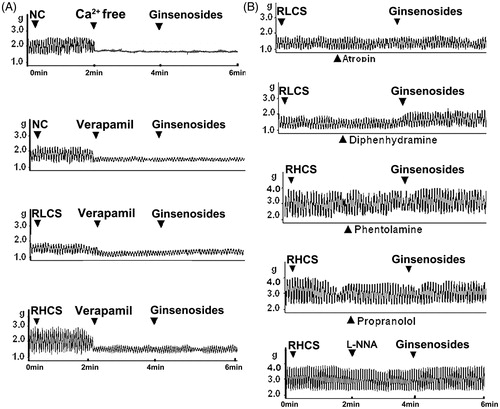
Table 1. Effects of ginsenosides on contractile amplitude of jejunal segment pretreated with receptor antagonist.
Figure 5. Effects of ginsenosides on myosin phosphorylation, MLCK contents, mRNA expression, and MLCK contents (A) the phosphorylation of 20-kDa regulatory light chain subunit of myosin (p-MLC20), (B) the protein content of myosin light chain kinase (MLCK) and (C) the expression of MLCK mRNA in the normal control group (NC), constipation-prominent group (CP), diarrhea-prominent group (DP), 30 mg/kg ginsenosides treated CP group (CP + C) and 30 mg/kg ginsenosides treated DP group (DP + C). All the data represent mean ± SEM from four independent experiments; **p < 0.01 compared with the NC.
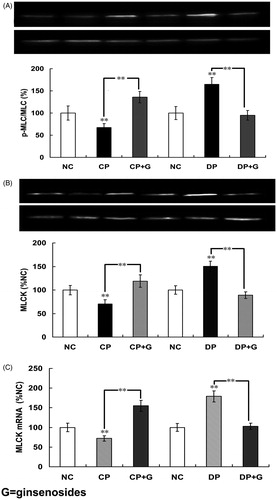
Table 2. Alleviating effects of ginsenosides on constipation and diarrhea symptoms.
