Figures & data
Figure 1. Comparison of binding mode of β-sitosterol in the active site pocket of human pancreatic α-amylase.
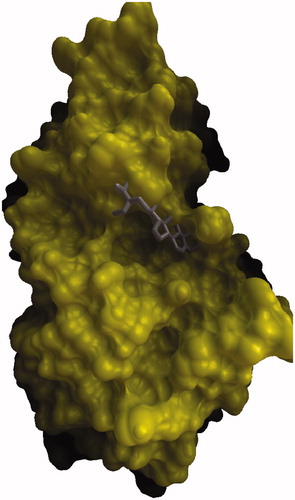
Figure 2. Hydrogen bond interactions between β-sitosterol and aminoacid residues in the active site pocket of human pancreatic α-amylase.
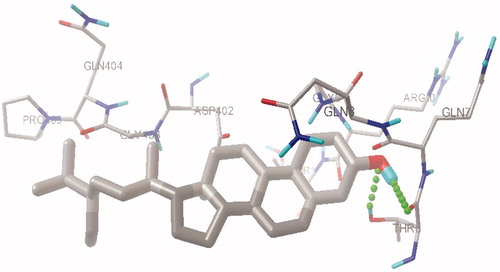
Figure 3. Hydrogen bond interactions between acarbose and aminoacid residues in the active site pocket of human pancreatic α-amylase.
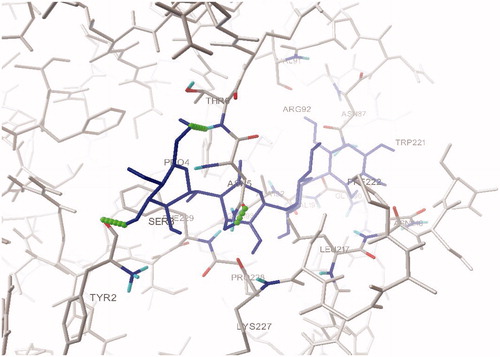
Table 1. Effect of ethyl acetate fraction and subfractions of Pterospermum acerifolium bark on changes in blood glucose level during OGTT.
Table 2. Effect of ethyl acetate fraction and subfractions of Pterospermum acerifolium bark on changes in blood glucose level.
Table 3. Effect of ethyl acetate fraction and subfractions of Pterospermum acerifolium bark on body weight changes and glycosylated Hb.
Table 4. Effect of ethyl acetate fraction and subfractions of Pterospermum acerifolium bark on levels of serum liver enzymes.
Table 5. Effect of ethyl acetate fraction and subfractions of Pterospermum acerifolium bark on levels of lipid parameters.
Table 6. Effect of ethyl acetate fraction and subfractions of Pterospermum acerifolium bark on levels of antioxidant status.
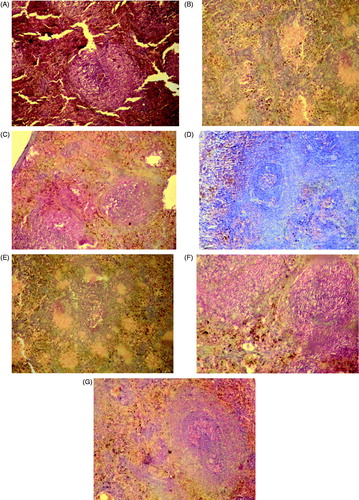
Figure 4. Histopathological observation of experimental rats pancreas after 30 days of treatment (A) normal control; (B) diabetic control; (C) diabetic animals treated with PABEF (400 mg/kg); (D) diabetic animals treated with PABE1 (30 mg/kg); (E) diabetic animals treated with PABE2 (30 mg/kg); (F) diabetic animals treated with PABE3 (30 mg/kg); (G) diabetic animals treated with glibenclamide (600 µg/kg).