Figures & data
Figure 1. Effect of GPE on HT-29 cell viability. The cells were treated with GPE at 6.25–100 µg/ml for 24, 48, and 72 h. The cell viability was determined by the resazurin reduction assay. The data represent the means ± S.E. of three independent experiments. *p < 0.05, **p < 0.01.
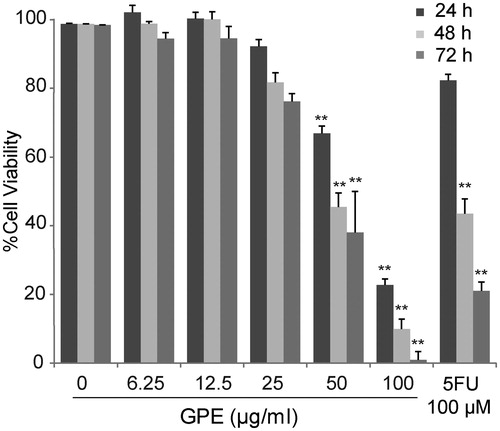
Figure 2. GPE induces apoptosis in HT-29 cells. The cells were treated with GPE 25–100 µg/ml for 24 h. The treated cells were stained with Annexin V-FITC/PI and analyzed by flow cytometry. (a) Representative flow cytometric analysis. (b) The percentages of cells labeled with annexin V+/PI− (early apoptotic cells), annexin V+/PI+ (late apoptotic cells), annexin V−/PI+ (necrotic cells), and total cell death were plotted. Data are the mean values ± S.E. of three independent experiments. *p < 0.05, **p < 0.01.
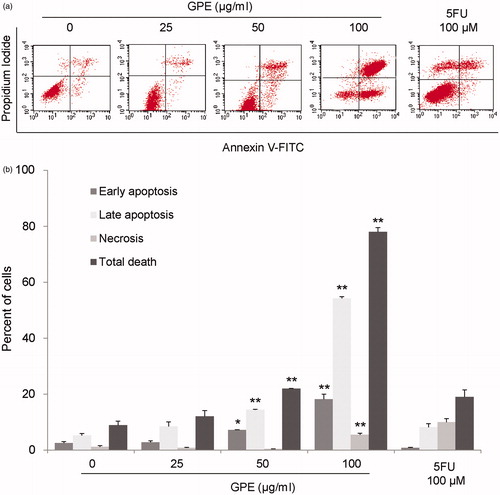
Figure 3. Antiproliferative effects of GPE against HT-29 cells. The cells were treated with GPE (25–100 µg/ml) for 24 h. Cell proliferation was measured by cell counting using an automated cell counter. The data represent the means ± S.E. of three independent experiments. *p < 0.05, **p < 0.01.
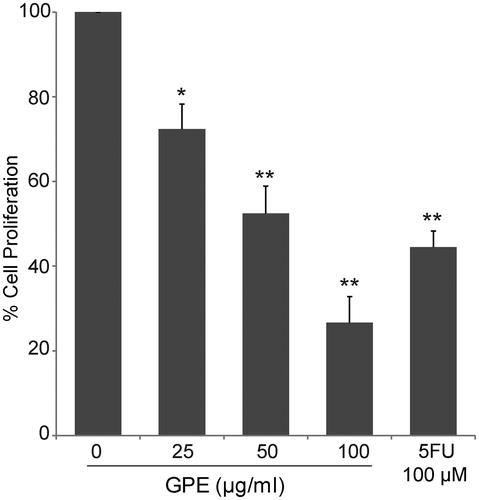
Figure 4. Distribution of HT-29 cells in the cell-cycle phases after treatment with GPE. HT-29 cells were treated with GPE 50 and 100 µg/ml for 1 h and further incubated in fresh medium for 24 h. Analysis of cells in the cell cycle was performed by fixing and staining the treated cells with PI followed by fluorescence flow cytometry. (a) Representative flow cytometric analysis. (b) The percentage of cells in G1, S, and G2M phases. Values are means ± S.E. of three independent experiments. *p < 0.05, **p < 0.01.
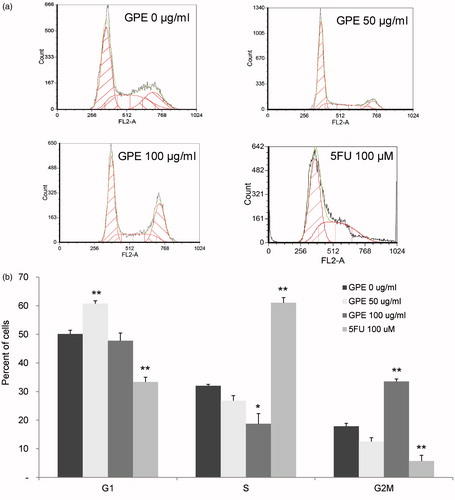
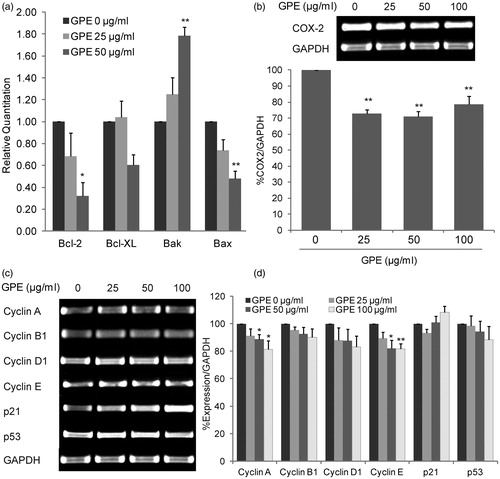
Figure 5. GPE alters the expression of BCL-2 family, COX-2, and cell-cycle regulators mRNA. (a) Expression of BCL-2 family genes by quantitative RT-PCR. The mRNA expression levels of BAK, BAX, BCL-2, and BCL-XL of HT-29 cells treated with GPE 25 and 50 µg/ml for 24 h were determined. The value is given as relative copy number normalized to the endogenous control GAPDH and 0.2% DMSO control. (b) COX-2 expression in HT-29 cells treated with GPE for 24 h. Expression of COX-2 was determined by RT-PCR. The percentage of PCR product of the COX-2 gene was normalized with the GAPDH and compared with the expression levels of the 0.2% DMSO control. (c) The HT-29 cells were treated with GPE for 1 h and incubated with fresh medium without GPE for additional 24 h. Expression of cell-cycle regulators were determined by RT-PCR. Representative bands of PCR products of the cell-cycle regulatory genes were shown. (d) The percentage of PCR product of the cell-cycle regulatory genes. The data represent the means ± S.E. of three independent experiments. *p < 0.05, **p < 0.01.