Figures & data
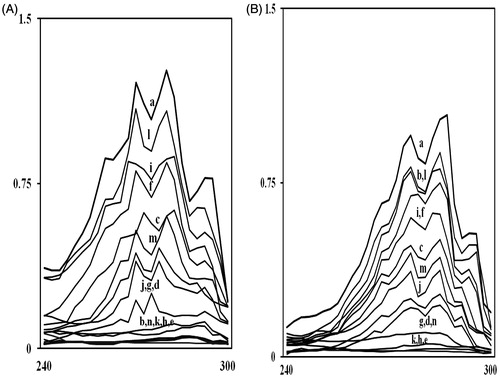
Figure 2. UV spectrophotometric sterol profiles of representative fluconazole-susceptible (A) and fluconazole-resistant (B) Candida strains. Strains were grown for 16 h in Sabouraud dextrose broth containing MIC/4, MIC/2, and MIC values of mint EO (curves c, d, and e); carvone (curves f, g, and h); menthol (curves i, j, and k), and menthone (curves l, m, and n), respectively. Curve ‘a’ is control (untreated cells) while curve ‘b’ shows positive control (FLC). Sterols were extracted from the cells and spectral profiles between 240 and 300 nm were determined.