Figures & data
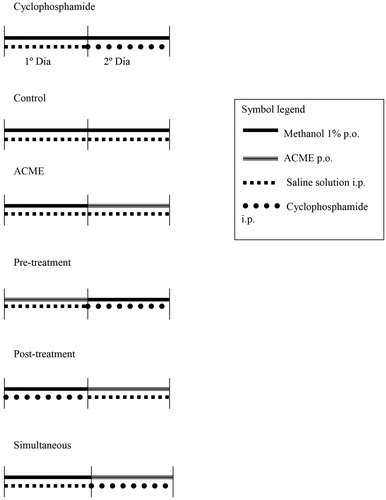
Figure 1. Treatments to evaluate the mutagenicity and antimutagenicity of A. crassiflora methanolic extract (ACME). Groups: negative control = distilled water 120 h; MMS = aqueous solution of methyl methanesulfonate; pre-treatment (48 h ACME + 48 h MMS), simultaneous simple (48 h distilled water + 48 h of association of ACME and MMS), simultaneous with pre-incubation (48 h distilled water + 48 h MMS with ACME pre-incubated for 1 h at 37 °C in the oven), post-treatment (48 h MMS + 48 h ACME), continuous (48 h ACME + 48 h MMS) without washing the seeds.
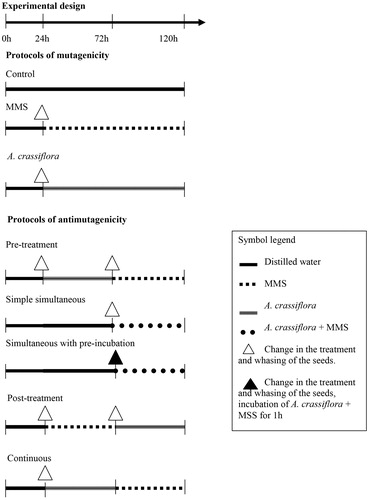
Figure 3. Effect of oral administration of ACME on carrageenan-induced paw edema in mice. Animal received ACME (30, 100, or 300 mg/kg, p.o.), dexamethasone (DEX – 1 mg/kg, s.c.) or vehicle and after 1 h, an intraplantar injection of carrageenan (300 µg/paw) was performed. In (A), the inhibition induced by 30, 100, and 300 mg/kg of ACME and DEX in paw edema (µm) at 4 h after carrageenan injection is shown. In (B), bars show the effect of different doses of 30, 100, and 300 mg/kg of ACME and DEX in increasing of myeloperoxidase (MPO) activity at 6 h after carrageenan injection is shown ACME and DEX. The bars express the mean ± SE of five animals, compared with the vehicle (V) versus the treated group. *p < 0.05, **p < 0.01, one-way ANOVA followed by the Student–Newman–Keuls test.
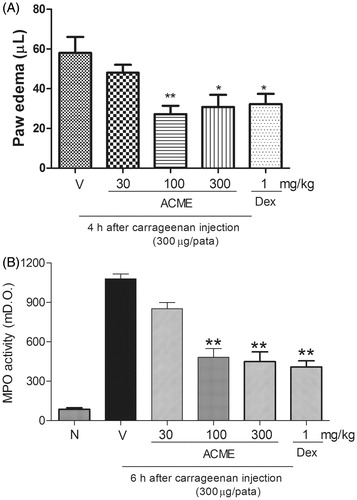
Figure 4. Effects of ACME on total leukocytes (A) and protein extravasation (B) induced by carrageenan in the pleural cavity of mice. Animal received the oral treatment with ACME (100 or 300 mg/kg), or vehicle, and after 1 h, they received an intrapleural injection of Cg (100 μL of a 1% solution/cavity). Control animals received only the vehicles and Naïve animal did not received carrageenan or ACME treatment. Animals were killed after Cg injection. The bars express the mean ± SEM of five animals, compared with the vehicle (V) versus the treated group. **p < 0.01, ***p < 0.001, one-way ANOVA followed by the Student–Newman–Keuls test. #p < 0.001.
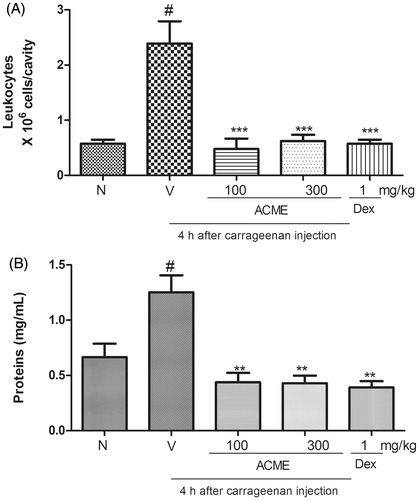
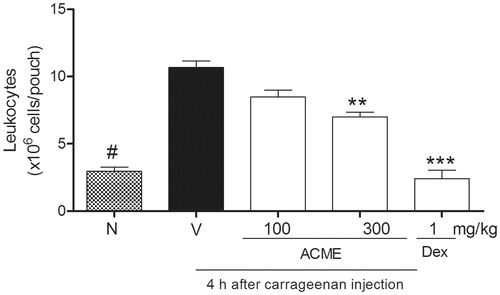
Figure 5. Effect of ACME on carrageenan-induced leukocyte migration and plasma leakage into the air pouch. Mice (n = 5) were pretreated 1 h before with ACME (10–300 mg/kg p.o.), dexamethasone (1 mg/kg, s.c., diluted in saline) or vehicle. Pouches were washed with PBS-containing heparin. Cells were counted and plasma leakage was analyzed. Results are expressed as cell 106/cavity. **p < 0.01, ***p < 0.001, #p < 0.001, compared with the vehicle group. Difference between groups were analyzed by analysis of variance (one-way ANOVA) followed by the Newman–Keuls test.