Figures & data
Figure 1. HPLC chromatograms of DOX in different samples. (A) DOX solution; (B) DOX in plasma sample; (C) DOX in heart sample; (D) DOX in liver sample; (E) DOX in spleen sample; (F) DOX in lung sample; (G) DOX in kidney sample; (H) DOX in tumor sample.
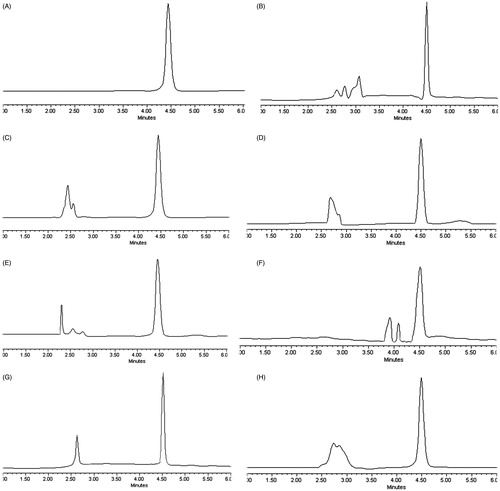
Table 1. Calibration curve of DOX in plasma and different tissues.
Table 2. Accuracy and precision of the method for determination of DOX contents.
Table 3. Recovery of the method for determination of DOX contents.
Figure 2. Mean DOX concentration–time curves in plasma after intravenous administration of free DOX, DOX/P(HB-HO) NPs and DOX/FA-PEG-P(HB-HO) NPs to tumor-bearing mice (n = 3) ((A) 0–48 h; (B) 0–12 h).
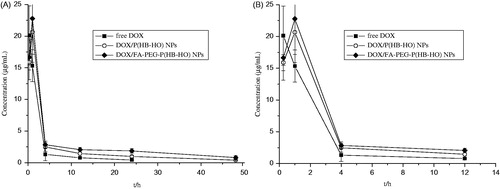
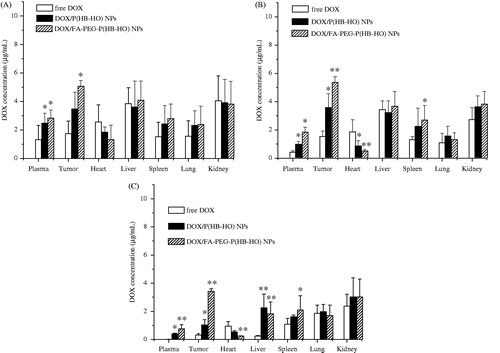
Figure 3. Biodistribution of free DOX, DOX/P(HB-HO) NPs and DOX/FA-PEG-P(HB-HO) NPs in blood, heart, liver, spleen, lung, kidney, and tumor at different times ((A) 4 h, (B) 24 h, (C) 48 h) after intravenous administration to tumor-bearing mice (n = 3). *p < 0.05 compared with free DOX; **p < 0.01 compared with free DOX.