Figures & data
Table 1. Effect of treatment with withanolide-rich fraction (WRF) on APAP-induced alteration of serum levels of albumin, direct bilirubin, total bilirubin, ALP, AST and ALT in rats.
Table 2. Effect of treatment with withanolide-rich fraction (WRF) on APAP-induced alteration in serum cholesterol, LDH, HDL, LDL and TG in rats.
Figure 1. Cyclic voltammograms analysis of rat liver homogenate in phosphate buffer saline. (A) The background (phosphate buffer alone) has been subtracted to estimate the peak anodic current (ip) i.e. total antioxidant potential. (B) Rat liver tissue, the superimposed voltammograms of (1) normal, (2) Silymarin (25 mg/kg)-treated, (3) WRF (200 mg/kg)-treated, (4) WRF (10 mg/kg)-treated, (5) WRF (50 mg/kg)-treated, (6) APAP-treated and (7) blank (phosphate buffer alone).
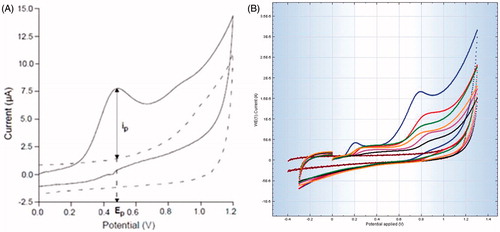
Table 3. Effect of withanolide-rich fraction (WRF) treatment on APAP-induced alteration on antioxidant parameters in rat liver.
Figure 2. Effect of WRF treatment on APAP-induced alteration in reverse transcriptase analysis of mRNA expression of TNF-α (A), IL-1β (B), iNOs (C) and COX-II (D).
Data are expressed as mean ± SEM and analyzed by one-way ANOVA followed by Dunnett’s test. *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to control group and #p < 0.05, ##p < 0.01, ###p < 0.001 as compared to normal group.
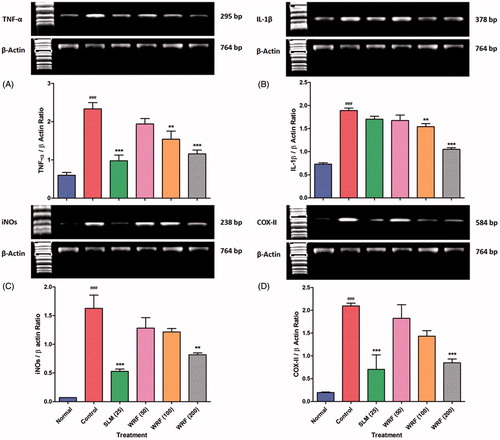
Figure 3. Effect of WRF treatment on APAP-induced pathological alteration in rat liver. Photomicrograph of sections of liver of normal (A), APAP-treated (B), Silymarin (25 mg/kg)-treated (C), WRF (50 mg/kg)-treated (D), WRF (100 mg/kg)-treated (E) and WRF (200 mg/kg)-treated (E) rats. Inflammatory infiltration (red arrow), congestion (yellow arrow), pyknosis (green arrow) and necrosis (black arrow). H&E staining at 40 X and 100 X (inset).
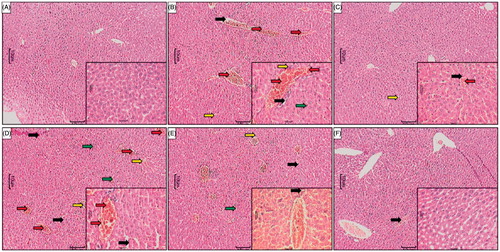
Table 4. Effect of withanolide-rich fraction (WRF) on APAP-induced histology alterations in rat liver.
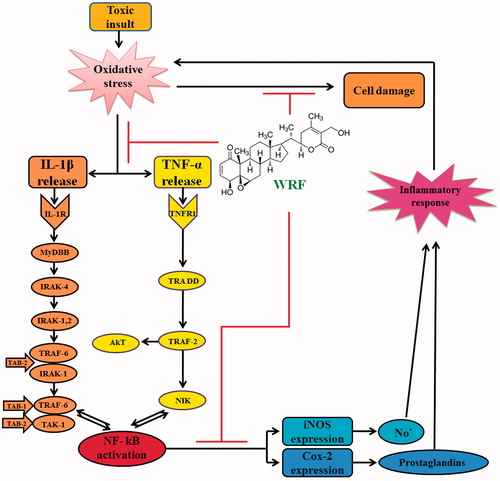
Figure 4. Scheme showing the proposed mechanisms of hepatoprotective activity of WRF. Toxic exposure could induce oxidative stress in the liver due to its metabolization into highly reactive free radicals. Oxidative stress triggers the IL-1β and TNF-α release from Kupffer cells and injured hepatocytes, which further activates NF-kB, allowing its nuclear translocation. Consequently, NF-kB stimulates the expression of iNOS and COX-II at the level of transcription, translation, and the enzyme level. The final products of iNOS and COX-II, NO- and prostaglandins, respectively, contributes to nitrosative stress and, on the other hand, initiate the cascade of the inflammatory response in the injured liver. Inflammation, in turn, is associated with the release of highly reactive oxygen and nitrogen species from inflammatory cells, further exacerbating oxidative and nitrosative stress. WRF prevents oxidative damage, as indicated by the decrease in lipid peroxidation, and improves the antioxidant status. Furthermore, WRF suppresses the inflammatory response by down-regulating the pro-inflammatory cascade initiated by TNF-α and IL-1β and attenuates nitrosative stress by the iNOS inhibition.