Figures & data
Scheme 1. Template condensation of 1,8-diaminonaphthalene and glyoxal in the presence of trivalent metal salts. M = Cr(III), Mn(III), Fe(III), X = Cl−, NO3−, CH3COO−.
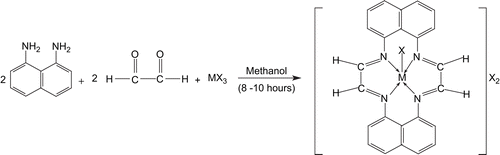
Table 1. Analytical data for trivalent chromium, manganese, and iron complexes derived from 1,8-diaminonaphthalene and glyoxal.
Figure 1. Thermogravimetric analysis curves. (a) TGA/DTG curve for [Cr(C24H16N4)Cl]Cl2 complex. (b) TGA/DTG curve for [Fe(C24H16N4)Cl]Cl2 complex.
![Figure 1. Thermogravimetric analysis curves. (a) TGA/DTG curve for [Cr(C24H16N4)Cl]Cl2 complex. (b) TGA/DTG curve for [Fe(C24H16N4)Cl]Cl2 complex.](/cms/asset/33efa5b1-d63c-4446-b25c-1b4ad2ef73d9/ienz_a_393447_f0002_b.gif)
Figure 2. Proposed structure of the complexes. M = Cr(III), Mn(III), Fe(III), X = Cl−, NO3−, CH3COO−.
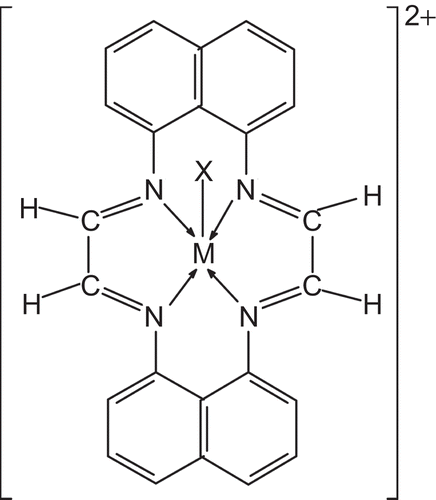
Figure 3. Comparison of MIC of compounds with standard antibiotics up to the concentration 32 μg/mL. a, Bacillus subtilis (MTCC 8509); b, Bacillus stearothermophilus (MTCC 8508); c, Pseudomonas putida (MTCC 121); d, Escherichia coli (MTCC 51). Chloramphenicol, streptomycin: standard antibiotics.
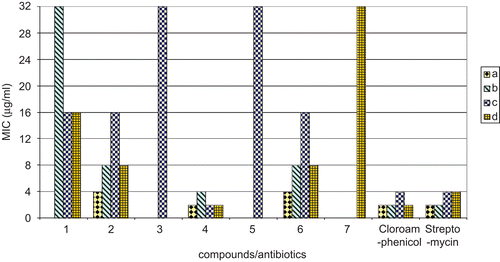
Table 2. Minimum inhibitory concentration (MIC) of complexes against test bacteria using agar dilution assay.
Figure 4. Comparison of percentage inhibition of compounds against fungal strains with standard drug cyclohexamide. f, Aspergillus flavus; g, Aspergillus niger. Cyclohexamide: standard drug.
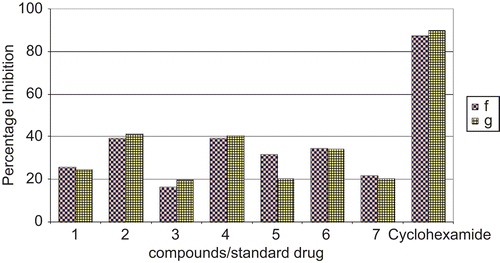
Table 3. Antifungal (percentage inhibition) activities of complexes against fungal strains (for concentration 100 μg/mL).
